Abstract
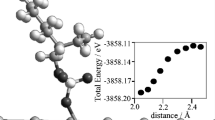
Calculation of coordination numbers showed that N-methylpyrrolidone (N-MP) forms stable 1: 4 metal-ligand complexes in sulfate cadmium-plating electrolytes. Methods of computer simulation and quantum-chemical calculations allowed establishing that thermodynamically stable Cd(II) complexes formed in the bulk of the sulfate electrolyte contain, apart from four N-MP molecules, four water molecules that provide additional stabilization of the complex due to formation of hydrogen bonds between the ligands. Electrochemical studies indicate the predominant participation of Cd(II) complexes in the electrode reaction. Their discharge is preceded by their slow dissociation. Hindrance of the process of Cd2+ ion electrore-duction from complex electrolytes results in improvement of the structure and quality of cathodic cadmium deposits.
Similar content being viewed by others
References
Dusek, B. and Kutek, F., Collect. Czech. Chem. Commun., 1975, vol. 40, p. 2569.
Choukroun, R. and Gervais, D., Inorg. Chim. Acta, 1978, vol. 26, no. 1, p. 17.
Eremin, Yu.G. and Martyshova, T.I., Zh. Neorg. Khim., 1970, vol. 15, no. 2, p. 350.
Kuznetsov, V.V., Skibina, L.M., Kuznetsova, E.F., et al., Zashch. Met., 2005, vol. 41, no. 5, p. 463.
Kuznetsov, V.V., Skibina, L.M., Kuznetsova, E.F., and Loskutnikova, I.N., Zashch. Met., 2006, vol. 42, no. 6, p. 632.
Skibina, L.M., Kuznetsov, V.V., Sokolenko, A.I., et al., Prot. Met. Phys. Chem. Surf., 2009, vol. 45, no. 1, p. 78.
Kravtsov, V.I., Elektrodnye protsessy v rastvorakh kompleksov metallov (Electrode Processes in Solutions of Metal Complexes), Leningrad: Izd-vo LGU, 1969.
Bek, R.Yu., Nechaev, E.A., and Kudryavtsev, N.T., Elektrokhimiya, 1967, vol. 3, p. 1465.
Delahay, P., New Instrumental Methods in Electrochemistry: Theory, Instrumentation, and Applications to Analytical And Physical Chemistry, New York: Interscience Publishers, 1954.
Casida, M., Recent Advances in Density Functional Methods, Singapore: World Scientific, 1995, Part 1.
Becke, A.D., J. Chem. Phys., 1993, vol. 98, p. 5648.
Burke, K., Perdew, J.P., and Wang, Y., Electronic Density Functional Theory: Recent Progress and New Directions, New York: Plenum, 1998.
Dunning, Jr., in Modern Theoretical Chemistry, Dunning, Jr., T.H., Hay, P.J., Schaefer, III, H.F., Eds., New York: Plenum, 1976, vol. 3, pp. 1–28.
McLean, A.D. and Chandler, G.S., J. Chem. Phys., 1980, vol. 72, p. 5639.
Halgren, T.A., Chem. Phys. Lett., 1977, vol. 49, p. 225.
Amovilli, C., Baron, U., Cammi, R., Cances, E., Cossi, M., Menucci, B., Pomelli, C.S., and Tomasi, J., Adv. Quant. Chem., 1998, vol. 32, p. 227.
Frisch, M.J. Gaussian 03, Revision E.01 / M.J. Frisch [and etc.].
Zhurko, G.A., ChemCraft Version 1.5 (build 275).
Galus, Z., Fundamentals of Electrochemical Analysis, New York: Wiley, 1994.
Kuznetsov, V.V. Skibina, L.M., et al., Zashch. Met., 2003, vol. 39, no. 2, p. 176.
Loshkarev, Yu.M., Zashch. Met., 1972, vol. 8, no. 2, p. 163.
Zakharov, M.S., Bakanov, V.I., and Pnev, V.V., Khronopotentsiometriya (Chronopotentiometry), Moscow: Khimiya, 1978.
Basolo, F. and Dzhonson, R., Khimiya koordinatsionnykh soedinenii (Chemistry of Coordination Compounds), Moscow: Mir, 1966.
Damaskin, B.B., Praktikum po elektrokhimii (Laboratory Course in Electrochemistry), Moscow: Vysshaya shkola, 1991.
Kukalenko, S.S., Usp. Khim., 1985, vol. 54, no. 7, p. 1152.
Gorokhovskaya, V.I. and Gorokhovskii, V.M., Praktikum po ostsillograficheskoi polyarografii (Laboratory Course in Oscillographic Polarography), Moscow: Vysshaya shkola, 1973.
Heirovsky, J. and Kuta, J., Osnovy polyarografii (Fundamentals of Polarography), Moscow: Mir, 1965.
Bond, A.M., Polyarograficheskie metody v analiticheskoi khimii (Polarographic Methods in Analytical Chemistry), Moscow: Khimiya, 1983.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.M. Skibina, I.V. Dorogan, A.A. Bumber, E.I. Burdina, 2013, published in Elektrokhimiya, 2013, Vol. 49, No. 2, pp. 138–145.
Rights and permissions
About this article
Cite this article
Skibina, L.M., Dorogan, I.V., Bumber, A.A. et al. Effect of complexation of cadmium ions with N-methylpyrrolidone on kinetics of their electroreduction in sulfate electrolyte. Russ J Electrochem 49, 124–130 (2013). https://doi.org/10.1134/S102319351302016X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102319351302016X