Abstract
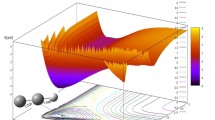
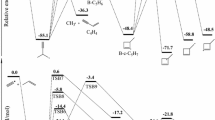
Potential energy surfaces (PESs) of the 1Al(1Σ + g ), 1B2 and 3B2 electronic states of CO2 have been computed as a function of the two bond distances and the bond angle. The calculations were based on the complete active space self consistent field (CASSCF) and multiconfigurational second-order perturbation theory (CASPT2) electronic structure models. From our calculations no crossing point between 1B2 and 3B2 states was found, but there is a crossing point located between 1B2 and 3A2 state on the PESs. The energy of the crossing point is lie 0.23 eV above the CO + O (3P), which is in agreement with the value of 0.27 eV on the experiment. This implies that the mechanism of the recombination of an oxygen atom with a carbon monoxide molecule: CO(X 1Σ+, ν) + O(3P)→3CO2*→1CO2*→CO(X 1Σ+, ν = 0) + O(1 D) may occur through the 3A2 state crossing the 1B2 state. The equilibrium geometries and adiabatic excitation energies of 1,3B2, 1,3A2 states of CO2 were reported and discussed in this paper, too.
Similar content being viewed by others
References
Z. Chen, F. Liu, B. Jiang, et al., J. Phys. Chem. Lett. 1, 1861 (2010).
I.-C. Lu, J. J. Lin, S.-H. Lee, et al., Chem. Phys. Lett. 382, 665 (2003).
A. Stolow and Y. T. Lee, J. Chem. Phys. 98, 2066 (1993).
R. L. Miller, S. H. Kable, P. L. Houston, et al., J. Chem. Phys. 96, 332 (1992).
Y. Matsumi, N. Shafer, K. Tonokura, et al., J. Chem. Phys. 95, 7311 (1991).
S. Y. Grebenshchikov, J. Chem. Phys. 137, 021101 (2012).
M. Braunstein and J. W. Duff, J. Phys. Chem. A 113, 10795 (2009).
A. L. Brunsvold, H. P. Upadhyaya, J. Zhang, et al., J. Phys. Chem. A 112, 2192 (2008).
H.-F. Chen and Y.-P. Lee, J. Phys. Chem. A 110, 12096 (2006).
Y. Matsumi, Y. Inagaki, G. P. Morley, et al., J. Phys. Chem. 100, 315 (1994).
M. Abe, Y. Inagaki, L. L. Springsteen, et al., J. Phys. Chem. 98, 12641 (1994).
D. R. Harding, J. R. E. Weston, and G. W. Flynn, J. Chem. Phys. 88, 3590 (1988).
R. G. Shortridge and M. C. Lin, J. Chem. Phys. 64, 4076 (1976).
O. F. Raper and W. B. DeMore, J. Chem. Phys. 40, 1053 (1964).
S. R. Kinnersly, Mol. Phys. 38, 1067 (1979).
V. Y. Simkin, A. I. Dement’ev, and V. I. Pupyshev, Russ. J. Phys. Chem. A 56, 1739 (1982).
M. C. Lin and S. H. Bauer, J. Chem. Phys. 50, 3377 (1969).
A. Spielfiedel, N. Feautrier, C. Cossart-Magos, et al., J. Chem. Phys. 97, 8382 (1992).
G. Karlström, L. Lingh, P.-Å. Malmqvist, et al., Comput. Mater. Sci. 28, 222 (2003).
R. B. Wattson and L. S. Rothman, J. Mol. Spectrosc. 119, 83 (1986).
C. Cossart-Magos, F. Launay, and J. E. Parkin, Mol. Phys. 75, 835 (1992).
R. N. Dixon, Proc. R. Soc. London A 275, 431 (1963).
N. W. Winter, C. F. Bender, and W. A. Goddard III, Chem. Phys. Lett. 20, 489 (1973).
M. Braunstein and J. W. Duff, J. Chem. Phys. 112, 2736 (2000).
M. A. A. Clyne and B. A. Thrush, Proc. R. Soc. London A 269, 404 (1962).
S. S. Xantheas and R. Klaus, Int. J. Quantum. Chem. 49, 409 (1994).
D.-Y. Hwang and A. M. Mebel, Chem. Phys. 256, 169 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Ma, Y., Peng, L., Zhang, H. et al. The potential energy surfaces of the ground and excited states of carbon dioxide molecule. Russ. J. Phys. Chem. 88, 2339–2347 (2014). https://doi.org/10.1134/S0036024414130287
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024414130287