Abstract
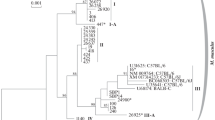
Variability of the nucleotide sequences of the second intron of the b1-chain of hemoglobin (Hbb-b1) and complete control region of mitochondrial DNA (D-loop) was studied in aboriginal and synanthropic populations of M. m. wagneri from Central Asia and M. m. gansuensis from South Siberia. A difference in the frequency of the Hbbw1 hemoglobin variant for natural and urban populations of mice was shown. All mice from natural habitats of studied areas have musculus type of mtDNA. Apparently, the substitution of taxon-specific mitochondrial haplotypes of wagneri, and gansuensis might occur due to the absorbing hybridization with nominate subspecies musculus, which is consistent with the results on nuclear DNA (Hbb-b1 gene) obtained in this work. Two differentiated haplogroups among aboriginal subspecies wagneri (d = 0.01), one of which included house mice from Turkmenistan, were discovered for the first time. This may indicate mtDNA introgression from synanthropic forms of Turkmenistan into natural populations of Kazakhstan mice. The type of mtDNA typical for the castaneus subspecies was detected in two individuals from the natural habitat of Kazakhstan and Turkmenistan; it had not been encountered in Central Asia before. It has been suggested that the gene flow of nuclear and mitochondrial genomes in microevolution processes in M. musculus is directed from the synanthropic forms towards wild populations.
Similar content being viewed by others
References
Zagorodniuk I.V. 1996. Taxonomic revision and diagnostics of the rodent genus Mus from eastern Europe: 1. Vestn. Zool. 30, 28–46.
Lavrenchenko L.A. 1994. Analysis of craniometric characters of house mice Mus musculus sensu lato (Rodentia, Muridae): A multivariate approach. Zool. Zh. 73, 169–177.
Korobitsyna K.V., Yakimenko L.V. 2004. Role and place of wagneri-like house mouse form (Rodentia, Muridae) in the fauna of Russia and neighboring countries. Zool. Zh. 83, 1018–1030.
Gromov I.M., Erbaeva M.A. 1995. Mlekopitayushchie fauny Rossii i sopredel’nykh territorii. Zaitseobraznye i gryzuny (Mammals of the Fauna of Russia and Bordering Territories: Lagomorphs and Rodents). St. Petersburg: Zool. Inst. Ross. Akad. Nauk.
Fortuyn D.A.B. 1931. Mus musculus and Mus wagneri compared: 1. The number of tailrings. Genetics. 16, 160–167.
Marshall J. 1998. Identification and Scientific Names of Eurasian House Mice and Their European Allies, Subgenus Mus (Rodentia, Muridae). Springfield, VA: Kinko’s.
Yakimenko L.V., Korobitsyna K.V., Frisman L.V., et al. 2003. Cytogenetics and systematics of house mice in Russia and neighboring countries. In: Problemy evolyutsii (Problems of Evolution). Vladivostok: Dal’nauka, pp. 62–89.
Frisman L.V. 1988. Protein polymorphism of house mice: A look at the systematics and settlement centers. In: Evolyutsionnye issledovaniya. Vavilovskie temy (Evolutionary Studies: Vavilov’s Themes). Vladivostok: Dal’nevost. Otd. Akad. Nauk SSSR, pp. 94–109.
Yonekawa H., Tsuda K., Tsuchiya K., et al. 2003. Genetic diversity, geographic distribution and evolutionary relationships of Mus musculus subspecies based on polymorphisms of mitochondrial DNA. In: Problemy evolyutsii (Problems of Evolution), vol. 5. Kryukov A.P., Yakimenko L.V. Eds. Vladivostok: Dal’nauka, pp. 90–108.
Spiridonova L.N., Korobitsyna K.V., Yakimenko L.V., Bogdanov A.S. 2008. Genetic differentiation of subspecies of the house mouse Mus musculus and their taxonomic relationships inferred from RAPD-PCR data. Russ. J. Genet. 44, 732–739.
Kawashima T., Miyashita N. B. Wang Ch., et al. 1991. A new haplotype of the β-globin gene complex, Hbb w1 in Chinese wild mouse. Jpn. J. Genet. 6 491–500.
Ueda Y., Miyashita N., Imai K., et al. 1999. Nucleotide sequences of the mouse globin beta gene cDNAs in a wild derived new haplotype Hbb w1. Mammal. Genome. 10, 879–882.
Kawashima T., Miyashita K., Tsuchiya K., et al. 1995. Geographical distribution of the Hbb haplotypes in the Mus musculus subspecies in Eastern Asia. Jpn. J. Genet. 70, 17–23.
Frisman L.V., Korobitsyna K.V., Yakimenko L.V., Munteanu A.I., Moriwaki K. 2011. Genetic variability and the origin of house mouse from the territory of Russia and neighboring countries. Russ. J. Genet. 47, 590–602.
Maniatis T., Fritsch E.F., Sambrook J. 1982. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press.
Sato J.J., Tsuru Y., Hirai K., et al. 2006. Further evidence for recombination between mouse hemoglobin beta b1 and b2 genes based on the nucleotide sequences of intron, UTR and intergenic spacer regions. Genes Genet. Syst. 81, 201–209.
Prager E.M., Sage R.D., Gyllensten U., et al. 1993. Mitochondrial DNA sequence diversity and the colonization of Scandinavia by house mice from East Holstein. Biol. J. Linn. Soc. 50, 85–122.
Boneld J.K., Smith K.F., Staden R. 1995. A new DNA sequence assembly program. Nucleic Acids Res. 23, 4992–4999.
Tamura K., Peterson D., Peterson N., et al. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony method. Mol. Biol. Evol. 28, 2731–2739.
Librado P., Rozas J. 2009. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 25, 1451–1452.
Felsenstein J. 1985. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 39, 783–791.
Bandelt H.-J., Forster P., Röhl A. 1999. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 16, 37–48.
Rogers A.R., Harpending H. 1992. Population growth makes waves in the distribution of pairwise genetic differences. Mol. Biol. Evol. 9, 552–569.
Kotenkova E.V. 2002. Hybridization of synantropic house mice species and its role in evolution. Usp. Sovrem. Biol. 122, 580–593.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.N. Spiridonova, 2014, published in Molekulyarnaya Biologiya, 2014, Vol. 48, No. 1, pp. 89–98.
Rights and permissions
About this article
Cite this article
Spiridonova, L.N. Introgression of nuclear and mitochondrial DNA markers of Mus musculus musculus to aboriginal populations of wild mice from Central Asia (M. m. wagneri) and South Siberia (M. m. gansuensis). Mol Biol 48, 75–83 (2014). https://doi.org/10.1134/S0026893314010142
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893314010142