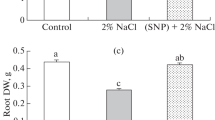
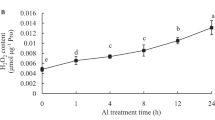
Abstract
With the enhancement of aluminum stress, the content of chlorophyll in wheat seedlings (Triticum aestivum L.) decreased dramatically. At 0.2 mM AlCl3, the chlorophyll content halved. The aluminum-induced decrease in chlorophyll content could be alleviated by exogenous nitric oxide donor, sodium nitroprusside (SNP) in a dose-dependent manner. Treatment with SNP dramatically promoted the activities of superoxide dismutase, catalase, ascorbate peroxidase and increased the proline content, whereas it decreased hydrogen peroxide and malondialdehyde and maintained the level of soluble protein as compared with water controls. Therefore, NO donor enhanced the antioxidant capacity in wheat seedlings under aluminum stress.
Similar content being viewed by others
Abbreviations
- APX:
-
ascorbate peroxidase
- CAT:
-
catalase
- MDA:
-
malondialdehyde
- ROS:
-
reactive oxygen species
- SNP:
-
sodium nitroprusside
- SOD:
-
superoxide dismutase
References
Ma, J.F., Ryan, P.R., and Delhaize, E., Aluminium Tolerance in Plants and the Complexing Role of Organic Acids, Trends Plant Sci., 2001, vol. 6, pp. 273–278.
Barceló, J. and Poschenrieder, C., Fast Root Growth Responses Root Exudates and Internal Detoxification as Clues to the Mechanisms of Aluminium Toxicity and Resistance: A Review, Environ. Exp. Bot., 2002, vol. 48, pp. 75–92.
Kochian, L.V., Cellular Mechanism of Aluminium Toxicity and Resistance in Plants, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1995, vol. 46, pp. 237–260.
Doncheva, S., Amenós, M., Poschenrieder, C., and Barceló, J., Root Cell Patterning: A Primary Target for Aluminium Toxicity in Maize, J. Exp. Bot., 2005, vol. 56, pp. 1213–1220.
Ezaki, B., Gardner, R.C., Ezaki, Y., and Matsumoto, H., Expression of Aluminum-Induced Genes in Transgenic Arabidopsis Plants Can Ameliorate Aluminum Stress and/or Oxidative Stress, Plant Physiol., 2000, vol. 122, pp. 657–665.
Richards, K.D., Schott, E.J., Sharma, Y.K., Davis, K.R., and Gardner, R.C., Aluminum Induces Oxidative Stress Genes in Arabidopsis thaliana, Plant Physiol., 1998, vol. 116, pp. 409–418.
Heath, R.L., The Biochemistry of Ozone Attack on the Plasma Membrane of Plant Cells, Rec. Adv. Phytochem., 1987, vol. 21, pp. 29–54.
Ezaki, B., Tsugita, S., and Matsumoto, H., Expression of a Moderately Anionic Peroxidase Is Induced by Aluminum Treatment in Tobacco Cells: Possible Involvement of Peroxidase Isozymes in Aluminum Ion Stress, Physiol. Plant., 1996, vol. 96, pp. 21–28.
Delledonne, M., Zeier, J., Marocco, A., and Lamb, C., Signal Interactions between Nitric Oxide and Reactive Oxygen Intermediates in the Plant Hypersensitive Disease Resistance Response, Proc. Natl. Acad. Sci. USA, 2001, vol. 98, pp. 13 454–13 459.
Bogdan, C., Nitric Oxide and the Regulation of Gene Expression, Trends Cell Biol., 2001, vol. 11, pp. 66–75.
De Pinto, M.C., Tommasi, F., and Gara, L.D., Changes in the Antioxidant Systems as Part of the Signaling Pathway Responsible for the Programmed Cell Death Activated by Nitric Oxide and Reactive Oxygen Species in Tobacco Bright-Yellow 2 Cells, Plant Physiol., 2002, vol. 130, pp. 698–708.
Leshem, Y.Y., Wills, R.B.H., and Ku, V.V.V., Evidence for the Function of the Free Radical Gas — Nitric Oxide (NO) — as an Endogenous Maturation and Senescence Regulating Factor in Higher Plants, Plant Physiol. Biochem., 1998, vol. 36, pp. 825–833.
Guo, F.Q. and Crawford, N.M., Arabidopsis Nitric Oxide Synthase 1 Is Targeted to Mitochondria and Protects against Oxidative Damage and Dark-Induced Senescence, Plant Cell, 2005, vol. 17, pp. 3436–3450.
Hung, K.T., Chang, C.J., and Kao, C.H., Paraquat Toxicity Is Reduced by Nitric Oxide in Rice Leaves, J. Plant. Physiol., 2002, vol. 159, pp. 159–166.
Zhang, H., Shen, W.B., and Xu, L.L., Effects of Nitric Oxide on the Germination of Wheat Seeds and Its Reactive Oxygen Species Metabolisms under Osmotic Stress, Acta Bot. Sinica, 2003, vol. 45, pp. 901–905.
Wang, Y.S. and Yang, Z.M., Nitric Oxide Reduces Aluminum Toxicity by Preventing Oxidative Stress in the Roots of Cassia tora L., Plant Cell Physiol., 2005, vol. 46, pp. 1915–1923.
Hoagland, D.R. and Arnon, D.I., The Water-Culture. Method for Growing Plants without Soil, California Agricultural Experimental Station Circular, Berkeley: CA: Univ. California, 1950.
García-Limones, C., Hervás, A., Navas-Cortés, J.A., Jiménez-Diaz, R.M., and Tena, M., Induction of an Antioxidant Enzyme System and Other Oxidative Stress Markers Associated with Compatible and Incompatible Interactions between Chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. Ciceris, Physiol. Mol. Plant. Pathol., 2002, vol. 61, pp. 325–337.
Ruan, H.H., Shen, W.B., Ye, M.B., and Xu, L.L., Protective Effects of Nitric Oxide on Salt Stress-Induced Oxidative Damage to Wheat (Triticum aestivum L.) Leaves, Chin. Sci. Bull., 2002, vol. 47, pp. 677–681.
Bradford, M.M., A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding, Anal. Biochem., 1976, vol. 72, pp. 248–254.
Nield, J., Redding, K., and Hippler, M., Remodeling of Light-Harvesting Protein Complexes in Chlamydomonas in Response to Environmental Changes, Eukaryot. Cell, 2004, vol. 3, pp. 1370–1380.
Mehta, R.A., Fawcett, T.W., Porath, D., and Mattoo, A.K., Oxidative Stress Causes Rapid Membrane Translocation and In Vivo Degradation of Ribulose-1,5-Biphosphate Carboxylase/Oxygenase, J. Biol. Chem., 1992, vol. 267, pp. 2810–2816.
Blokhina, O., Virolainen, E., and Fagerstedt, K.V., Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review, Ann. Bot., 2003, vol. 91, pp. 179–194.
Beligni, M.V. and Lamattina, L., Nitric Oxide Counteracts Cytotoxic Processes Mediated by Reactive Oxygen Species in Plant Tissues, Planta, 1999, vol. 208, pp. 337–344.
Jiang, M.Y., Yang, W.Y., and Xu, J., Active Oxygen Damage Effect on Chlorophyll Degradation in Rice Seedlings under Osmotic Stress, Acta Bot. Sinica, 1994, vol. 36, pp. 289–295.
Bowler, C. and Fluhr, R., The Role of Calcium and Activated Oxygens as Signals for Controlling Cross-Tolerance, Trends Plant Sci., 2000, vol. 5, pp. 241–246.
Neill, S.J., Desikan, R., Clarke, A., Hurst, R.D., and Hancock, J.T., Hydrogen Peroxide and Nitric Oxide as Signaling Molecules in Plants, J. Exp. Bot., 2002, vol. 53, pp. 1237–1247.
Zaninotto, F., Camera, S.L., Polverari, A., and Delledonne, M., Cross Talk between Reactive Nitrogen and Oxygen Species during the Hypersensitive Disease Resistance Response, Plant Physiol., 2006, vol. 141, pp. 379–383.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © H. Zhang, Y.H. Li, L.Y. Hu, S.H. Wang, F.Q. Zhang, K.D. Hu, 2008, published in Fiziologiya Rastenii, 2008, Vol. 55, No. 4, pp. 523–528.
This text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Zhang, H., Li, Y.H., Hu, L.Y. et al. Effects of exogenous nitric oxide donor on antioxidant metabolism in wheat leaves under aluminum stress. Russ J Plant Physiol 55, 469–474 (2008). https://doi.org/10.1134/S1021443708040067
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443708040067