Abstract
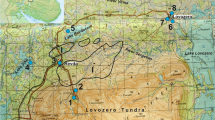
Sampling data on subsoil and surface water near a storage site of low-activity liquid radioactive wastes, receiving neutralized uranium-containing pulps are given. Thermodynamic calculations are used to describe the behavior of major polluting and petrogenic components in the drainage system, allowing the occurrence forms of elements in solution and/or solid phase to be determined. The results suggest a positive effect of natural geochemical barriers hampering the process of uranium migration into natural waters. Over-all, the technogenic-natural system under consideration was found to effectively cope with the removal of uranium-containing compounds from industrial water.
Similar content being viewed by others
References
Gas’kova, O.L., Geochemical Features and Physicochemical Parameters of Hypergene Processes in Technogenic Zones, Doctoral (Geol.-min.) Dissertation, Novosibirsk: Inst. of Geology and Mineralogy, SB RAS, 2005.
Gas’kova, O.L., Semiempirical Model for the Description of Sorption Equilibria on Clay Mineral Surfaces, Geochem. Int. 2009, no. 6, pp. 611–622.
Iskra, A.A. and Bakhurov, V.G., Estestvennye radionuklidy v biosfere (Natural Radinuclides in Biosphere), Moscow: Energoizdat, 1981.
Karbonaty: mineralogiya i geokhimiya (Carbonates: Mineralogy and Geochemistry), Reader, R.J., Ed., Moscow: Mir, 1987.
Kovalev, V.P., Mel’gunov, S.V., Puzankov, Yu.M., and Raevskii, V.P., Predotvrashchenie neupravlyaemogo rasprostraneniya radionuklidov v okruzhayushchuyu sredu (Prevention of Uncontrollable Spread of Radionuclides in the Environment), Novosibirsk: NITs OIGGM SO RAN, 1996.
Krainov, S.R. and Zakutin, V.P., Geochemical-Ecological State of Groundwater in Russia (The Causes and Trends in Groundwater Chemistry Changes), Geokhimiya, 1994, no. 3, pp. 312–329.
Metody geokhimicheskogo modelirovaniya i prognozirovaniya v gidrogeologii (Methods of Geochemical Modeling and Prediction in Hydrogeology), Krainov, S.R., Shvarov, Yu.V., Grichuk, D.V., et al., Eds., Moscow: Nedra, 1988.
Osnovy gidrogeologii (Fundamentals of Hydrogeology), Shvartsev, S.L., Ed., Novosibirsk: Nauka, 1982, vol. 2.
Rukovodstvo po khimicheskomu analizu poverkhnostnykh vod sushi (Guide on Chemical Analysis of Continental Surface Water), Semenov, A.D., Ed., Leningrad: Gidrometeoizdat, 1977.
Tkachev, V.V., Behavior and Occurrence Forms of Plutonium in Subsoil Waters, Extended Abstract of Cand. Sci. (Chem.) Dissertation, Moscow: GEOKhI RAN, 2008.
Shvarov, Yu.V., Algorithmization of the Numeric Equilibrium Modeling of Dynamic Geochemical Processes, Geochem. Int., 1999, no. 6, pp. 571–576.
Shvartsev, S.L., Gidrogeokhimiya zony giperegeneza (Hydrochemistry of Hypergene Zone), Moscow: Nedra, 1998.
Ball, J.W. and Nordstrom, D.K., User’s manual for WATEQ4F, with revised thermodynamic database. Menlo Park: U.S. Geological Survey, 1991–1992.
Bradbury, M.H. and Baeyens, B., Modelling the Sorption of Mn(II), Co(II), Ni(II), Zn(II), Cd(II), Eu(III), Am(III), Sn(IV), Th(IV), Np(V) and U(VI) on Montmorillonite: Linear Free Energy Relationships and Estimates of Surface Binding Constants for Some Selected Heavy Metals and Actinides, Geochim. Cosmochim. Acta, 2005, vol. 69, no. 4, pp. 875–892.
Choppin, G.R., Actinide Speciation in Aquatic System, Marine Geochem, 2006, vol. 99,Is. 1–4, pp. 83–92.
Grenthe, I., Fuger, J., and Konings, R.J.M., Chemical Thermodynamics of Uranium, Amsterdam: Elsevier, 1992, vol. 1.
Gu, B., Brooks, S.C., Roh, Y., and Jardine, P.M., Geochemical Reactions and Dynamics during Titration of a Contaminated Groundwater with High Uranium, Aluminum, and Calcium, Geochim. Cosmochim. Acta, 2003, vol. 67, no. 15, pp. 2749–2761.
Hummel, W., Berner, U., Curti, E., et al., NAGRA (National Cooperative for the Disposal of Radioactive Waste) /PSI Chemical Thermodynamic Data Base 01/01. Nagra Technical Report NTB 02-16. Wettingen: PSI, 2002.
Hummel, W., Glaus, M.A., and Van Loon, L.R., Trace Metal-Humate Interactions. II. The “Conservative Roof” Model and Its Application, Applied Geochem, 2000, vol. 15,Is. 7, pp. 975–1001.
Kirishima, A., Onishi, Y., Sato, N., and Tochiyama, O., Thermodynamic Study on the U(VI) Complexation with Dicarboxylates by Calorimetry, Radiochchim. Acta, 2008, vol. 96,Is. 9–11, pp. 581–589.
Author information
Authors and Affiliations
Additional information
Original Russian Text © O.L. Gas’kova, A.E. Boguslavskii, T.G. Sirotenko, 2011, published in Vodnye Resursy, 2011, Vol. 38, No. 5, pp. 553–563.
Rights and permissions
About this article
Cite this article
Gas’kova, O.L., Boguslavskii, A.E. & Sirotenko, T.G. Geochemical composition of natural waters near a storage site of low-activity radioactive wastes. Water Resour 38, 597–607 (2011). https://doi.org/10.1134/S0097807811050071
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0097807811050071