Abstract
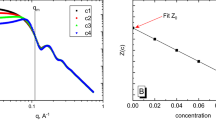
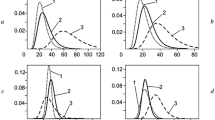
It has been established by small-angle X-ray scattering and sedimentation analysis that aqueous solutions of poly(aluminum hydroxychloride) are disperse systems comprising two types of nanoparticles considerably different in size. The volume content, molecular weights, radii of gyration, characteristic size, specific surface, fractal dimensions, and shape of poly(aluminum hydroxychloride) nanoparticles have been determined. The particles of poly(aluminum hydroxychloride) have been shown to be rather stable entities, and the addition of HCl has practically no effect on their molecular weight or structural characteristics.
Similar content being viewed by others
References
E. S. Lukin, “Modern High-Density Oxide Ceramic Materials with a Controlled Microstructure,” Ogneupory Tekh. Keram., No. 4, 2–13 (1996).
O. E. Litmanovich, G. V. Marmuzov, A. A. Litmanovich, and I. M. Papisov, “Selectivity of the Interactions between Copper Nanoparticles and Macromolecules of Polyelectrolyte and Nonionogenic Polymers,” Vysokomol. Soedin., Ser. A 45(9), 1533–1543 (2003) [Polym. Sci., Ser. A 45 (9), 906–916 (2003)].
S. A. Ozerin, S. A. Zav’yalov, and S. N. Chvalun, “Synthesis, Structure, and Properties of Metal-Polymer Nanocomposites Based on Silver and Poly-p-Xylylene,” Vysokomol. Soedin., Ser. A 41(11), 1993–1999 (2001) [Polym. Sci., Ser. A 41 (11), 1171–1176 (2001)].
S. S. Ivanchev, A. M. Mesh, N. Reichelt, S. Ya. Khaikin, A. Hesse, and S. V. Myakin, “Preparation of Nanocomposites by Alkoxysilane Hydrolysis in a Polypropylene Matrix,” Vysokomol. Soedin., Ser. A 44(6), 996–1001 (2002) [Polym. Sci., Ser. A 44 (6), 623–627 (2002)].
I. A. Novakov, F. S. Radchenko, and I. M. Papisov, “Formation of Polycomplexes Based on Polyacrylamide and Aluminum Salts,” Vysokomol. Soedin., Ser. A 45(8), 1340–1349 (2003) [Polym. Sci., Ser. A 45 (8), 805–808 (2003)].
I. A. Novakov, F. S. Radchenko, A. S. Pastukhov, and I. M. Papisov, “The Properties of Aqueous Solutions of Polymer-Colloid Complexes of Polyacrylamide with Poly(aluminum hydroxychloride),” Vysokomol. Soedin., Ser. A 47(1), 73–80 (2005) [Polym. Sci., Ser. A 47 (1), 57–60 (2005)].
A. V. Volkov, M. A. Moskvina, A. B. Zezin, A. L. Volynksii, and N. F. Bakeev, “The Effect of a Polymer Matrix on the Structure of Nanocompositions Containing Cadmium Sulfide,” Vysokomol. Soedin., Ser. A 45(2), 283–291 (2003) [Polym. Sci., Ser. A 45 (2), 166–173 (2003)].
C. Y. Brinker and G. W. Scherer, Sol-Gel Science: the Physics and Chemistry of Sol-Gel Processing (Academic, Boston, 1990).
O. A. Shilova and V. V. Shilov, “Nanocomposite Oxide and Hybride Organic-Inorganic Materials Obtained by the Sol-Gel Method: Synthesis, Properties, and Applications,” Nanosyst., Nanomater., Nanotechnol. 1(1), 9–83 (2003).
G. G. Kagramanov, P. V. Kholkin, and E. A. Lukashov, “Simulation of the Sol-Gel Process of the Formation of Selective Layers of Ceramic Membranes,” Ogneupory Keram., No. 5, 2–13 (2001).
A. R. Barron and S. J. Obrey, “Supra-Molecular Alumoxanes,” US Patent No. 6322890 (November 27, 2001).
O. B. Pavlova-Verevkina, V. F. Kargin, and Yu. E. Roginskaya, “Preparation and Properties of Stable Aluminum Hydroxide Sols: Morphology of Highly Dispersed Aluminum Hydroxide (Pseudoboehmite),” Kolloidn. Zh. 55(3), 127–137 (1993).
V. V. Nazarov, E. K. Valesyan, and N. G. Medvedkova, “The Effect of Electrolytes on the Aggregation Stability of Boehmite Hydrosols,” Kolloidn. Zh. 61(1), 91–94 (1999) [Colloid J. 61 (1), 82–85 (1999)].
M. A. Fedotov, O. P. Krivoruchko, and R. A. Buyanov, “Interaction of Initial Anionic Salts with Products of Hydrolytic Polymerization of Aqua Ions,” Izv. Akad. Nauk SSSR, Ser. Khim., No. 10, 2183–2186 (1977).
O. P. Krivoruchko and R. A. Buyanov, Thermodynamics and Structure of Hydroxocomplexes in Solutions (Nauka, Leningrad, 1980) [in Russian].
M. G. Osmolovskii, I. A. Zvereva, and D. I. Tarasenko, “An Uncharged Cluster-the Main Structural Element of Amorphous Hydroxides,” in Proceedings of the Sixth All-Russia (International) Conference “Physicochemistry of Ultradispersed (Nano-) Systems,” Moscow Institute of Engineering Physics (MIFI), Moscow, Russia, 2003 (Moscow, 2003).
M. E. Shishniashvili, V. A. Kargin, and A. L. Batsanadze, “Preparation of Aluminum Hydroxy Salts and Investigation of Their Properties,” Zh. Fiz. Khim. 21(3), 391–396 (1947).
E. A. Levitskii and V. N. Maksimov, “On the Composition of Products of Hydrolysis in Solutions of Aluminum Chloride,” Dokl. Akad. Nauk SSSR 141(4), 865–868 (1961).
I. M. Solomentseva, N. G. Gerasimenko, and V. V. Teselkin, “Size and Density Characteristics for Products of Hydrolysis of Aluminum Hydroxychlorides,” Khim. Tekhnol. Vody 15(11–12), 719–725 (1993).
O. S. Zakharchenko, E. A. Litmanovich, F. S. Radchenko, A. S. Pastukhov, A. B. Zezin, I. A. Novakov, and V. A. Kabanov, “Photon Correlation Spectroscopic Study of the Aggregative Stability of Colloidal Particles of Aluminum Pentahydroxide Chloride,” Kolloidn. Zh. 68(4), 467–471 (2006) [Colloid J. 68 (4), 425–429 (2006)].
O. P. Krivoruchko, V. N. Kolomiichuk, and R. A. Buyanov, “Investigation of the Formation of Aluminum(III) Hydroxides Using the Small-Angle X-Ray Scattering Method,” Zh. Neorg. Khim. 30(2), 306–310 (1985).
I. A. Novakov, N. U. Bykadorov, S. S Radchenko, Yu. N. Kargin, V. F. Mokhov, O. K. Zhokhova, A. I. Parkhomenko, and P. I. Otchenashev, RF Patent No. 2083495, Byull. Izobret., No. 19 (July 10, 1997).
D. I. Svergun, “Determination of the Regularization Parameter in Indirect-Transform Methods Using Perceptual Criteria,” J. Appl. Crystallogr. 25, 495–503 (1992).
D. I. Svergun and L. A. Feigin, Structure Analysis by Small-Angle X-Ray and Neutron Scattering (Nauka, Moscow, 1986; Plenum, New York, 1987).
A. Guinier, “New Method for the Small-Angle Scattering Data,” Ann. Phys. (Paris) 12, 161–237 (1939).
D. I. Svergun, “Restoring Low-Resolution Structure of Biological Macromolecules from Solution Scattering Using Simulated Annealing,” Biophys. J. 76(6), 2879–2886 (1999).
V. V. Volkov and D. I. Svergun, “Uniqueness of Ab Initio Shape Determination in Small-Angle Scattering,” J. Appl. Crystallogr. 36 Part 3 (1), 860–864 (2003).
A. N. Ozerin, A. M. Muzafarov, A. I. Kuklin, A. Kh. Islamov, V. I. Gordelyi, G. M. Ignat’eva, V. D. Myakushev, L. A. Ozerina, and E. A. Tatarinova, “Determination of the Shape of Dendrimer Macromolecules in Solutions from Small-Angle Neutron Scattering Data,” Dokl. Akad. Nauk, Ser. Khim. 395(4), 487–490 (2004) [Dokl. Chem. 395 (Part 2), 59–62 (2004)].
A. N. Ozerin, D. I. Svergun, V. V. Volkov, A. I. Kuklin, V. I. Gordelyi, A. Kh. Islamov, L. A. Ozerina, and D. S. Zavorotnyuk, “The Spatial Structure of Dendritic Macromolecules,” J. Appl. Crystallogr. 38, 996–1003 (2005).
A. N. Ozerin, A. M. Muzafarov, L. A. Ozerina, D. S. Zavorotnyuk, I. B. Meshkov, O. B. Pavlova-Verevkina, and M. A. Beshenko, “Reproduction of the Shape of Nanodisperse Particles from Small-Angle X-Ray Scattering Data without Use of a Priori Information,” Dokl. Akad. Nauk 411(1), 71–74 (2006) [Dokl. Chem. 411 (Part 1), 202–205 (2006)].
M. B. Kozin and D. I. Svergun, “Automated Matching of High- and Low-Resolution Structural Models,” J. Appl. Crystallogr. 34, 33–41 (2001).
P. V. Konarev, M. V. Petoukhov, and D. I. Svergun, “MASSHA-A Graphics System for Rigid-Body Modelling of Macromolecular Complexes against Solution Scattering Data,” J. Appl. Crystallogr. 34, 527–532 (2001).
J. E. Martin and A. J. Hurd, “Scattering from Fractals,” J. Appl. Crystallogr. 20, 61–78 (1987).
G. Porod, “The X-Ray Small-Angle Scattering of Close-Packed Colloid Systems,” Kolloid. Z. 125, 109–122 (1952).
G. Beaucage, “Approximations Leading to a Unified Exponential/Power-Law Approach to Small-Angle Scattering,” J. Appl. Crystallogr. 28, 717–728 (1995).
G. Beaucage, “Small-Angle Scattering from Polymeric Mass Fractals of Arbitrary Mass-Fractal Dimension,” J. Appl. Crystallogr. 29, 134–146 (1996).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.S. Ozerin, F.S. Radchenko, G.I. Timofeeva, I.A. Novakov, 2009, published in Rossiiskie nanotekhnologii, 2009, Vol. 4, Nos. 1–2.
Rights and permissions
About this article
Cite this article
Ozerin, A.S., Radchenko, F.S., Timofeeva, G.I. et al. A study of structural and molecular weight characteristics of poly(aluminum hydroxychloride) nanoparticles by small-angle X-ray scattering and sedimentation analysis. Nanotechnol Russia 4, 93–101 (2009). https://doi.org/10.1134/S1995078009010108
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995078009010108