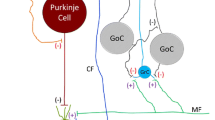
Abstract
Gamma-aminobutyric acid (GABA) and nitric oxide are two key-transmitters in cerebellar nuclei, the major output of cerebellar circuitry. The aims of this study were to investigate the effects of acute intra-cerebellar administration of ethanol (20 mM) on extra-cellular levels of GABA and on the NMDA-induced nitric oxide (NO) production using microdialysis in the rat. We also studied: (i) the effects of a pre-administration of DNQX, a specific antagonist of AMPA receptors, on NO production, (ii) the effects of a pre-administration of 7-NI (7-nitroindazole, an inhibitor of neuronal nitric oxide synthase NOS) and APV (D-2-amino-5-phosphonovaleric acid, a specific blocker of the NMDA type glutamate receptors) on the actions of alcohol/NMDA on glutamate receptors, and (iii) thein vivo interaction between DNQX, ethanol and NMDA receptor activation. We found that ethanol decreased the amount of extra-cellular GABA, and that this effect was counterbalanced by administration of tiagabine 1 mg/kg, a potent inhibitor of GAT-1 GABA transporter, given by the i.p. route. In loco administration of NMDA increased the levels of NO, as previously reported. A pre-administration of DNQX (500 microM) increased significantly the production of NO up to toxic levels, as well as ethanol administration. A preadministration of 7-NI or APV reduced significantly the amounts of NO when NMDA and alcohol were infused simultaneously. The combination of ethanol with DNQX was associated with a marked enhancement of the concentrations of NO. The activity of GAT-1 in cerebellar nuclei and around this target, including in glial cells expressing GAT-1 activated by ambient GABA, seems to be spared by ethanol. Tiagabine could be considered as a candidate for future investigational treatments of acute ethanol-induced dysfunction of cerebellar nuclei. We found a potentiation of the production of NO when AMPA antagonists are given simultaneously to ethanol. The hypothesis of AMPA neurotoxicity, which has convincing arguments during chronic exposure, is challenged in this model of acute cerebellar nuclear toxicity of alcohol.
Similar content being viewed by others
References
Pentney R. Alcohol toxicity in the cerebellum: Fundamental aspects. In: Manto M, Pandolfo M, editors. The cerebellum and its disorders. Cambridge: Cambridge University Press; 2002.
Bhave SV, Ghoda L, Hoffman PL. Brain-derived neurotrophic factor mediates the anti-apoptotic effect of NMDA in cerebellar granule neurons: Signal transduction cascades and site of ethanol action. J Neurosci. 1999;19:3277–86.
Bloom FE, Siggins GR. Electrophysiological action of ethanol at the cellular level. Alcohol. 1987;4:331–7.
Iorio KR, Reinlib L, Tabakoff B, Hoffman PL. Chronic exposure of cerebellar granule cells to ethanol results in increased N-methyl-D-aspartate receptor function. Mol Pharmacol. 1992;41:1142–8.
Hauser KF, Khurdayan VK, Goody RJ, Nath A, Saria A, Pauly JR. Selective vulnerability of cerebellar granule neuroblasts and their progeny to drugs with abuse liability. Cerebellum. 2003;2:184–95.
Lovinger DM. Excitotoxicity and alcohol-related brain damage. Alcohol Clin Exp Res. 1993;17:19–27.
Strahlendorf JC, Acosta S, Miles R, Strahlendorf HK. Choline blocks AMPA-induced dark cell degeneration of Purkinje neurons: potential role of the 7 nicotinic receptor. Brain Res. 2001;901:71–8.
Garthwaite G, Garthwaite J. Mechanisms of AMPA neurotoxicity in rat brain slices. Eur J Neurosci. 1991;3:729–36.
Ericson M, Haythornthwaite AR, Yeh PW, Yeh HH. Brainderived neurotrophic factor mitigates chronic ethanolinduced attenuation of gamma-aminobutyric acid responses in cultured cerebellar granule cells. J Neurosci Res. 2003;73:722–30.
Nestoros JN. Ethanol specifically potentiates GABA-mediated neurotransmission in feline cerebral cortex. Science. 1980;209:708–10.
Reynolds JN, Prasad A, MacDonald JF. Ethanol modulation of GABA receptor-activated Cl-currents in neurons of the chick, rat and mouse central nervous system. Eur J Pharmacol. 1992;224:173–81.
Palmer MR, Hoffer BJ. GABAergic mechanisms in the electrophysiological actions of ethanol on cerebellar neurons. Neurochem Res. 1990;15:145–51.
Chen L, Yung WH. Effects of the GABA-uptake inhibitor tiagabine in rat globus pallidus. Exp Brain Res. 2003;152: 263–9.
Dalby NO. GABA-level increasing and anticonvulsant effects of three different GABA uptake inhibitors. Neuropharmacology. 2000;39:2399–2407.
Barakat L, Bordey A. GAT-1 and reversible GABA transport in Bergmann glia in slices. J Neurophysiol. 2002;88:1407–19.
Smolders I, Sarre S, Michotte Y, Ebinger G. The analysis of excitatory, inhibitory and other amino acids in rat brain microdialysates using microbore liquid chromatography. J Neurosci Meth. 1995;57:47–53.
Castro-Alamancos MA, Torres-Aleman I. Long-term depression of glutamate-induced gamma-aminobutyric acid release in cerebellum by insulin-like growth factor I. Proc Natl Acad Sci USA. 1993;90:7386–90.
Stengard K, Tham R, O’Connor WT, Hoglund G, Ungerstedt U. Acute toluene exposure increases extracellular GABA in the cerebellum of rat: a microdialysis study. Pharmacol Toxicol. 1993;73:315–18.
Bert A, Favale D, Jego G, et al. Rapid and precise method to locate microdialysis probe implantation in the rodent brain. J Neurosci Meth. 2004;140:53–7.
Rosina A, Morara S, Provini L. GAT-1 developmental expression in the rat cerebellar cortex: Basket and pinceau formation. Neuroreport. 1999;10:1613–18.
Morara S, Brecha NC, Marcotti W, Provini L, Rosina A. Neuronal and glial localization of the GABA transporter GAT-1 in the cerebellar cortex. Neuroreport. 1996;7:2993–6.
Ribak CE, Tong WM, Brecha NC. Astrocytic processes compensate for the apparent lack of GABA transporters in the axon terminals of cerebellar Purkinje cells. Anat Embryol (Berl). 1996;194:379–90.
Durkin MM, Smith KE, Borden LA, Weinshank RL, Branchek TA, Gustafson EL. Localization of messenger RNAs encoding three GABA transporters in rat brain: An in situ hybridization study. Brain Res Mol Brain Res. 1995;33:7–21.
Limatola C. Neurotrophic effects of AMPA. Cerebellum. 2004;3:2–10.
Tanii Y, Nishikawa T, Hashimoto A, Takahashi K. Stereoselective antagonism by enantiomers of alanine and serine of phencyclidine-induced hyperactivity, stereotypy and ataxia in the rat. J Pharmacol Exp Therap. 1994;269:1040–8.
Saigoh K, Matsui K, Takahashi K, Nishikawa T, Wada K. The stereo-specific effect of D-serine ethylester and the Dcycloserine in ataxic mutant mice. Brain Res. 1998;808:42–7.
Ogawa M, Shigeto H, Yamamoto T, Oya Y, Wada K, Nishikawa T, Kawai M. D-cycloserine for the treatment of ataxia in spinocerebellar degeneration. J Neurol Sci. 2003;210:53–6.
Navamani M, Morgan M, Williams RJ. Ethanol modulates N-methyl-D-aspartate-evoked arachidonic acid release from neurones. Eur J Pharmacol. 1997;340:27–34.
Miller B, Sarantis M, Traynelis SF, Attwell D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992;355:722–5.
Williams RJ, Murphy N, Glowinski J, Pr’emont J. Glucose regulates glutamate-evoked arachidonic acid release from cultured striatal neurones. J Neurochem. 1995;65:241–9.
Linden DJ. Phospholipase A2 controls the induction of shortterm versus long-term depression in the cerebellar purkinje neuron in culture. Neuron. 1995;15:1393–401.
Chan PH, Kerlan R, Fishman RA. Reductions of gammaaminobutyric acid and glutamate uptake and NaK-ATPase activity in brain slices and synaptosomes by arachidonic acid. J Neurochem. 1983;40:309–16.
Volterra A, Trotti D, Cassutti P, Tromba C, Salvaggio A, Melcangi RC, Racagni G. High sensitivity of glutamate uptake to extracellular free arachidonic acid levels in rat cortical synaptosomes and astrocytes. J Neurochem. 1992;59:600–6.
Grant KA, Vaverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176:289–96.
Hu XJ, Ticku MK. Chronic ethanol treatment upregulates the NMDA receptor function and binding in mammalian cortical neurons. Mol Brain Res. 1995;30:347–56.
Fataccioli V, Gentil M, Nordmann R, Rouach H. Inactivation of cerebellar nitric oxide synthase by ethanol in vitro. Alcohol Alcohol. 1997;32:683–91.
Baud O, Li J, Zhang Y, Neve RL, Volpe JJ, Rosenberg PA. Nitric oxide-induced cell death in developing oligodendrocytes is associated with mitochondrial dysfunction and apoptosis-inducing factor translocation. Eur J Neurosci. 2004;20:1713–26.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manto, M., Laute, MA. & Pandolfo, M. Depression of extra-cellular GABA and increase of NMDA-induced nitric oxide following acute intra-nuclear administration of alcohol in the cerebellar nuclei of the rat. Cerebellum 4, 230–238 (2005). https://doi.org/10.1080/14734220500243835
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1080/14734220500243835