Summary
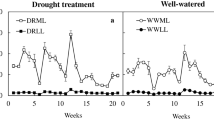
Ananas comosus (L.) Merr. var. Smooth Cayenne plants when grown in vitro under different temperature regimes developed as CAM or as C3 plants. The plants used in this study were developed from the lateral buds of the nodal etiolated stem explants cultured on Murashige and Skoog medium for 3 mo. The cultures were maintained under a 16-h photoperiod for different thermoperiods. With 28°C light/15°C dark thermoperiod, as compared with constant 28°C light and dark, pineapple plants had a succulence index two times greater, and also a greater nocturnal titratable acidity and phosphoenolpyruvate carboxylase (PEPCase) activity, indicating CAM-type photosynthesis. The highest abscisic acid (ABA) level occurred during the light period, 8 h prior to maximum PEPCase activity, while the indole-3-acetic acid (IAA) peak was found during the dark period, coinciding with the time of highest PEPCase activity. These plants were also smaller with thicker leaves and fewer roots, but had greater dry weight. Their leaves showed histological characteristics of CAM plants, such as the presence of greater quantities of chlorenchyma and hypoderm. In addition, their vascular system was more conspicuous. In contrast, under constant temperature (28°C light/dark) plants showed little succulence in the leaves. There was no significant acid oscillation and diurnal variation in PEPCase activity in these plants, suggesting the occurrence of C3 photosynthesis. Also, no diurnal variation in ABA and IAA contents was observed. The results of this study clearly indicate a role for temperature in determining the type of carbon fixation pathway in in vitro grown pineapple. Evidence that ABA and IAA participate in CAM signaling is provided.
Similar content being viewed by others
References
Arnon, D. I. Copper enzyme in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24:411–416; 1949.
Bartholomew, D. P.; Kadzimin, S. B. Pineapple. In: Alvin, P.T.; Kozeowski, T. T. eds. Ecophysiology of tropical crops. New York, NY: Academic Press; 1977:113–156.
Bartholomew, D. P.; Malézieux, E. P. Pineapple. In: Schaffer, B.; Andersen, P. C. eds. Handbook of environmental physiology of fruit crops, Vol. 2. Boca Raton: CRC Press; 1994:243–291.
Beltrán-Peña, E.; Aguilar, R.; Ortiz-López, A.; Dinkova, T. D.; Sánchez-de-Jiménez, E. Auxin stimulates S6 ribosomal protein phosphorylation in maize thereby affecting protein synthesis regulation. Physiol. Plant. 115:291–297; 2002.
Borland, A. M.; Griffiths, H. The regulation of citric acid accumulation and carbon recycling during CAM in Ananas comosus. J. Exp. Bot. 40:53–60; 1989.
Borland, A. M.; Hartwell, J.; Jenkins, G. I.; Wilkins, M. B.; Nimmo, H. G. Metabolic control overrides circadian regulation of phosphoenolpyruvate carboxylase kinase and CO2 fixation in crassulacean acid metabolism. Plant Physiol. 121:889–896; 1999.
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254; 1976.
Chardot, T. P.; Wedding, R. T. Regulation of Crassula argentea phosphoenolpyruvate carboxylase in relation to temperature. Arch. Biochem. Biophys. 293:292–297; 1992.
Chollet, R.; Vidal, J.; O'Leary, M. H. Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47:273–298; 1996.
Chu, C.; Dai, Z.; Ku, M. S. B.; Edwards, G. Induction of crassulacean acid metabolism in the facultative halophyte Mesembryanthemum crystallinum by abscisic acid. Plant Physiol. 93:1253–1260; 1990.
Cohen, J.D. Convenient apparatus for the generation of small amounts of diazomethane. J. Chromatogr. 303:193–196; 1984.
Endres, L.; Souza, B. M.; Mercier, H. In vitro nitrogen nutrition and pattern in bromeliads. In Vitro Cell. Dev. Biol. Plant 38:481–486; 2002.
Fahn, A.; Cutler, D. F. Xerophytes. Berlin: Gebrüder Borntraeger; 1992.
Friemert, V.; Heininger, D.; Kluge, M.; Ziegler, H. Temperature effects on malic-acid efflux from the vacuoles and on the carboxylation pathways in crassulacean acid metabolism plants. Planta 174:453–461; 1988.
Haag-Kerwer, A.; Franco, A. C.; Lüttge, U. The effect of temperature and light on gas exchange and acid accumulation in the C3-CAM plant Clusia minor L. J. Exp. Bot. 43:345–352; 1992.
Hendry, G. A.; Price, A. H. Stress indicators: chlorophylls and carotenoids. In: Hendry, G. A. F.; Grime, J. P., eds. Methods in comparative plant ecology. London: Chapman and Hall; 1993:150–152.
Karnovsky, M. J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 27:137–138; 1965.
Kluge, M.; Ting, I. P. Crassulacean acid metabolism: analysis of an ecological adaptation. Berlin: Springer-Verlag; 1978.
Leport, L.; Kandbinder, A.; Baur, B.; Kaiser, W. M. Diurnal modulation of phosphoenolpyruvate carboxylation in pea leaves and roots as related to tissue malate concentrations and to the nitrogen source. Planta 198:495–501; 1996.
Lüttge, L. Ecophysiology of crassulacean acid metabolism (CAM). Ann. Bot. 93:629–652; 2004.
Maldiney, R.; Leroux, B.; Sabbagh, I.; Sotta, B.; Sossountzov, L.; Miginiac, E. A biotin-avidin-based enzyme immunoassay to quantify three phytohormones: auxin, abscisic acid and zeatin-riboside. J. Immunol. Meth. 90:151–158; 1986.
Martin, C. E. Physiological ecology of the Bromeliaceae. Bot. Rev. 60:1–81; 1994.
Medina, E.; Lüttge, U.; Leal, F.; Ziegler, H. Carbon and hydrogen isotope ratios in bromeliads growing under different light environments in natural conditions. Bot. Acta 104:47–52; 1991.
Medina, E.; Ziegler, H.; Lüttge, U.; Trimborn, P.; Francisco, M. Light conditions during growth as revealed by 13C values of leaves of primitive cultivars of Ananas comosus, an obligate CAM species. Funct. Ecol. 8:298–305; 1994.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Neales, T. F.; Sale, P. J. M.; Meyer, C. P. Carbon dioxide assimilation by pineapple plants, Ananas comosus (L.) Merr. II. Effects of variation of the day/night temperature regime. Aust. J. Plant Physiol. 7:375–385, 1980.
Nioevola, C. C.; Mercier, H.; Majerowicz, N. Levels of nitrogen assimilation in bromeliads with different growth habits. J. Plant Nutr. 24:1387–1398; 2001.
Nimmo, H. G. The regulation of phosphoenolpyruvate carboxylase in CAM plants. Trends Plant Sci. 5:75–80; 2000.
O'Brien, T. P.; Feder, N.; McCully, M. E. Polychromatic staining of plant cell walls by toluidine blue O. Proplasma 59:368–373; 1965.
Osmond, B.; Maxwell, K.; Popp, M.; Robinson, S. On being thick: fathoming apparently futile pathways of photosynthesis and carbohydrate metabolism in succulent CAM plants. In: Bryant, J. A.; Burrell, M. N.; Kruger, N., eds. Plant carbohydrate biochemistry. Oxford: BIOS Scientific Publishers; 1999:183–200.
Ota, K.; Yamamoto, Y. Effects of different nitrogen sources and concentration on CAM photosynthesis in Kalanchöe blossfeldiana. J. Exp. Bot. 30:971–981; 1991.
Pérez, L.; Aguilar, R.; Sánchez-de-Jiménez, E. Effects of an exogenous auxin on maize tissues. Alteration of protein synthesis and phosphorylation. Physiol. Plant. 69:517–522; 1987.
Sayed, O. H. Crassulacean acid metabolism 1975–2000, a check list. Photosynthetica 39:339–352; 2001.
Sipes, D.; Ting, I. P. Crassulacean acid metabolism and modifications in Peperomia camptotricha. Plant Physiol. 77:59–63; 1985.
Taybi, T.; Cushman, J. C. Signaling events leading to crassulacean acid metabolism induction in the common ice plant. Plant Physiol. 121:545–555; 1999.
Taybi, T.; Cushman, J. C.; Borland, A. M. Environmental, hormonal and circadian regulation of crassulacean acid metabolism expression. Funct. Plant Biol. 29:669–678; 2002.
Taybi, T.; Sotta, B.; Gehrig, H.; Güşlü, S.; Kluge, M.; Brulfert, J. Differential effects of abscisic acid on phosphoenolpyruvate carboxylase and CAM operation in Kalanchoë blossfeldiana. Bot. Acta 108:240–246; 1995.
Teeri, J. A.; Tonsor, S. J.; Turner, M. Leaf thickness and carbon isotope composition in Crassulaceae. Oecologia 50:369–397; 1981.
Thomas, J.; McElwain, E. F.; Bohnert, H. J. Convergent induction of osmotic stress-responses: abscisic acid, cytokinin, and the effect of NaCl. Plant Physiol. 100:416–423; 1992.
Ting, I. P. Effects of ABA on CAM in Portulacaria afra. Photosynth. Res. 2:39–48; 1981.
Tomlinson, P. B. Commelinales—Zingiberales. In: Metcalfe, C. R., ed. Anatomy of the monocytoledons, vol. 3. London: Oxford University Press; 1969:193–294.
Zhu, J.; Bartholomew, D. J.; Goldstein, G. Effect of elevated carbon dioxide on the growth and physiological responses of pineapple, a species with crassulacean acid metabolism. J. Am. Soc. Hort. Sci. 122:233–237; 1997.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nievola, C.C., Kraus, J.E., Freschi, L. et al. Temperature determines the occurrence of CAM or C3 photosynthesis in pineapple plantlets grown in vitro . In Vitro Cell.Dev.Biol.-Plant 41, 832–837 (2005). https://doi.org/10.1079/IVP2005694
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2005694