Abstract
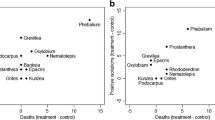
Fifteen native Western Australian legumes were assessed for their potential to biologically control Phytophthora cinnamomi. Biological control was assessed in a controlled situation, conducive to P. cinnamomi, and was based on susceptibility to the pathogen, ability to reduce soil inoculum, amount of asymptomatic root infection and ability for P. cinnamomi to effectively sporulate from asymptomatic ally infected roots. Acacia extensa, Acacia stenoptera and Acacia alata along with Acacia pulchella, were identified as species with the highest potential for biological control of P. cinnamomi. Acacia urophylla and Viminaria juncea exhibited the least potential for biological control; these are more likely to harbour the pathogen and provide a source of inoculum when conditions become conducive for P. cinnamomi growth and development. These findings have important implications for managing the rehabilitation of bauxite-mined P. cinnamomi-infested areas and severely disease-affected forest. By manipulating rehabilitation seed mix ratios, the density of legume species that suppress P. cinnamomi inoculum in the soil can be increased and the density of those that harbour the pathogen can be reduced. This could potentially contain the activity of P. cinnamomi soil inoculum in infested areas to protect susceptible species and enhance species diversity. Further research is required to ascertain the action of suppression before implementing control measures.
Similar content being viewed by others
References
Bais HP, Walker TS, Schweizer HP, Vivanco JM (2002) Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of Ocimum basilicum. Plant Physiology and Biochemisty 40, 983–995. doi: 10.1016/S0981-9428(02)01460-2
Broadbent P, Baker KF, Waterworth Y (1971) Bacteria and actinomycetes antagonistic to fungal root pathogens in Australian soils. Australian Journal of Biological Sciences 24, 925–944.
Burrows ND (1985) Reducing the abundance of Banksia grandis in the jarrah forest by the use of controlled fire. Australian Forestry 48, 63–70.
Burrows ND (1987) Fire caused bole damage to jarrah (Eucalyptus marginata) and marri (Eucalyptus calophylla). Department of Conservation and Land Management, Research Paper 3, Western Australia.
Colquhoun IJ, Hardy GEStJ (2000) Managing the risks of Phytophthora root and collar rot during bauxite mining in the Eucalyptus marginata (Jarrah) forest of Western Australia. Plant Disease 84, 116–127.
Dell B, Malajczuk N (1989) Jarrah dieback—a disease caused by Phytophthora cinnamomi. In ‘The jarrah forest. A complex mediterranean ecosystem’. (Eds B Dell, JJ Havel, N Malajczuk) pp. 67–87. (Kluwer Academic Publishers: Dordrecht, Netherlands)
D’Souza NK, Colquhoun IJ, Shearer BL, Hardy GEStJ (2004) The potential of five Western Australian native Acacia species for biological control of Phytophthora cinnamomi. Australian Journal of Botany 52, 267–279. doi: 10.1071/BT03089
Downer AJ, Menge JA, Pond E (2001) Association of cellulytic enzyme activities in Eucalyptus mulches with biological control of Phytophthora cinnamomi. Phytopathology 91, 847–855.
El-Tarabily KA, Sykes ML, Kurtbke ID, Hardy GEStJ, Barbosa AM, Dekker RFH (1996) Synergistic effects of a cellulase-producing Micromonospora carbonacea and an antibiotic-producing Streptomyces violascens on the suppression of Phytophthora cinnamomi root rot of Banksia grandis. Canadian Journal of Botany 74, 618–624.
Erwin DC, Bartnicki-Garcia S, Tsao PH (Eds) (1983) Phytophthora It’s Biology Taxonomy Ecology and Pathology. (The American Phytopathological Society: St Paul, MN)
Halsall DM (1978) A comparison of Phytophthora cinnamomi infection in Eucalyptus sieberi a susceptible species and Eucalyptus maculata a field resistant species. Australian Journal of Botany 26, 643–655.
Hüberli D, Tommerup IC, Hardy GESU (2000) False negative isolations or absence of lesions may cause mis-diagnosis of diseased plants infected with Phytophthora cinnamomi. Australasian Plant Pathology 29, 164–169.
Hüberli D, Tommerup IC, Dobrowolski MP, Calver MC, Hardy GEStJ (2001) Phenotypic variation in a clonal lineage of two Phytophthora cinnamomi populations from Western Australia. Mycological Researches, 105 1053–1064.
Komorek BM, Shearer BL, Blumberg MV, Fairman RG (1997) Potassium phosphite: effective chemical tool in the protection of native flora threatened by Phytophthora. In ‘Proceedings Australasian Plant Pathology Society 11th Biennial Conference’. Perth, Western Australia. Abstract.
Krupa S, Nylund J (1972) Studies on ectomycorrhizae of pine. Ill Growth inhibition of two root pathogenic fungi by volatile organic constituents of ectomycorrhizal root systems of Pinus silvestris L. European Journal of Forest Pathology 2, 88–94.
Malajczuk N( 1979) Biological suppression of Phytophthora cinnamomi in eucalypts and avocados in Australia. In ‘Soil-borne plant pathogens’. (Eds B Schippers and W Gams) pp. 635–652. (Academic Press: London, UK)
Malajczuk N, Nesbitt HJ, Glenn AR (1977) A light and electron microscope study of the interaction of soil bacteria with Phytophthora cinnamomi Rands. Canadian Journal of Microbiology 23, 1518–1525.
Marks GC, Cerra R (1991) Effects of propazine and chlorthal dimethyl on Phytophthora cinnamomi root disease of Pinus radiata seedlings and associated soil microflora. Soil Biology & Biochemistry 23, 157–164. doi: 10.1016/0038-0717(91)90129-8
Menge JA, McDonald V (2004) Management of soil microorganisms for the control of Phytophthora root rot. Phytopathology 94, S125.
Murray DIL (1987) Rhizosphere microorganisms from the jarrah forest of Western Australia and their effects on vegetative growth and sporulation in Phytophthora cinnamomi Rands. Australian Journal of Botany 35, 567–580.
Murray DIL, Darling DD, McGann LR (1985) Indirect effect of floristic composition on production of sporangia by Phytophthora cinnamomi in jarrah forest soils. Australian Journal of Botany 33, 109–113.
Nesbitt HJ, Glenn AR, Malajczuk N (1981) Effect of soil leachates on the release and motility of zoospores of Phytophthora cinnamomi Rands. Soil Biology & Biochemistry 13, 79–81. doi: 10.1016/0038-0717(81)90108-5
Phillips D, Weste G (1984) Field resistance in three native monocotyledon species that colonize indigenous sclerophyll forest after invasion by Phytophthora cinnamomi. Australian Journal of Botany 32, 339–352.
SAS (1989) ‘SAS/STAT Users Guide Version 6’. 4th edition. (SAS Institute: Cary, NJ)
Shea SR, Malajczuk N (1977) Potential for control of eucalypt dieback in Western Australia. In ‘Australian Nurserymen’s Association Limited Annual conference seminal papers’. Hobart, Australia, pp 13–19.
Shea SR, Malajczuk N, Kitt RJ (1976) Promotion of understorey native legumes — a possible method of control of Phytophthora cinnamomi in the northern jarrah forest of WA. In ‘Abstracts of papers. Second national plant pathology conference Brisbane May 12–14 1976’, Abstract 05. (The Australian Plant Pathology Society: Brisbane)
Shea SR, Gillen KJ, Kitt RJ (1978) Variation in sporangial production of Phytophthora cinnamomi Rand s on jarrah (Eucalyptus marginata Sm) forest sites with different understorey compositions. Australian Forest Research 8, 219–226.
Shearer BL, Bailey R (1989) The fight against jarrah dieback. Landscope 5, 38–44.
Shearer BL, Dillon M (1995) Susceptibility of plant species in Eucalyptus marginata forest to infection by Phytophthora cinnamomi. Australian Journal of Botany 43, 113–134.
Shearer BL, Dillon M (1996) Susceptibility of plant species in Banksia woodlands on the Swan coastal plain Western Australia to infection by Phytophthora cinnamomi. Australian Journal of Botany 44, 433–145.
Shearer BL, Tippett JT (1989) Jarrah dieback, the dynamics and management of Phytophthora cinnamomi in the jarrah (Eucalyptus marginata) forest of south-western Australia. Research Bulletin No. 3, November 1989, Department of Conservation and Land Management, Western Australia.
Shearer BL, Wills R, Stukely M (1991) Wildflower killers. Landscope 1, 28–34.
Shearer BL, Crane CE, Cochrane A (2004) Quantification of the susceptibility of the native flora of the South West Botanical Province, Western Australia, to Phytophthora cinnamomi. Australian Journal of Botany 52, 435–443. doi: 10.1071/BT03131
Smith IW, Marks GC (1983) Influence of Acacia spp on the control of Phytophthora cinnamomi root rot of Eucalyptus sieberi. Australian Forest Research 13, 231–240.
Smith IW, Marks GC, Featherston GR, Geary PW (1989) Effects of inter-planted wattles on the establishment of eucalypts planted on forest sites affected by Phytophthora cinnamomi. Australian Forestry 52, 74–81.
Tippett J, Malajczuk N (1979) Interaction of Phytophthora cinnamomi and a resistant host Acacia pulchella. Phytopathology 69, 765–772.
Tippett JT, Holland AA, Marks GC, O’Brien TP (1976) Penetration of Phytophthora cinnamomi into disease tolerant and susceptible eucalypts. Archives of Microbiology 108, 231–242. doi: 10.1007/BF00454847
Weste G, Cahill D (1982) Changes in root tissue associated with infection by Phytophthora cinnamomi. Phytopathology 103, 97–108.
Whitfield FB, Shea SR, Gillen KJ, Shaw KJ (1981) Volatile components from the roots of Acacia pulchella RBr and their effect on Phytophthora cinnamomi Rands. Australian Journal of Botany 29, 195–208.
Yin B, Scupham AJ, Menge JA, Borneman J (2004) Identifying microorganisms which fill a niche similar to that of the pathogen: a new investigative approach for discovering biological control organisms. Plant and Soil 259, 19–27. doi: 10.1023/B:PLSO.0000020944.45798.56
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D’Souza, N.K., Colquhoun, I.J., Sheared, B.L. et al. Assessing the potential for biological control of Phytophthora cinnamomi by fifteen native Western Australian jarrah-forest legume species. Australasian Plant Pathology 34, 533–540 (2005). https://doi.org/10.1071/AP05067
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1071/AP05067