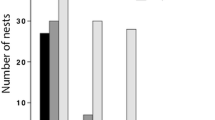
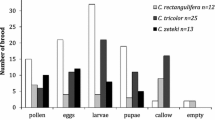
Abstract
It has been assumed that allodapine bees represent early stages in the evolution of social behaviour. Early studies suggested that sociality evolved from solitary forms, and that the solitary to social transition coincided with a transition from mass to progressive provisioning of brood. Recent studies challenge both of these assumptions, they suggest that: (i) Macrogalea replaces Halterapis + Compsomelissa as the sister group to all other genera; (ii) sociality is plesiomorphic for the tribe; and based on extended Halterapis research, (iii) there are no strictly solitary allodapine species and, therefore, no reversals to solitary living. Penalised likelihood dating of Bayesian inferred phylograms show allodapine lineages have an origin older than 40 Mya. The early origin of sociality in this tribe may explain the diverse array of social organization (and social parasitism) found in species across a range of clades, and the age of the group raises curious biogeographic scenarios.
Zusammenfassung
Einige Bienen und Wespen sind fakultativ sozial. Anders als Honigbienen, Ameisen und Termiten sind ihre reproduktiven Rollen nicht durch morphologische Kasten eingeschränkt. Alle Weibchen sind daher in der Lage ihre eigene Brut unabhängig aufzuziehen. Daher ist die entstehende Gruppendynamik (soziale Organisation) hoch flexibel und reicht von der solitären Lebensweise bis zu hochorganisierten (eusozialen) Gemeinwesen, wobei diese Unterschiede sowohl innerhalb einer Art als auch zwischen nahverwandten Arten auftreten.
Aus diesem Grund sind solche Organismen sehr gut für vergleichende Untersuchungen über altruistisches Verhalten und dessen Entstehung geeignet. Warum sollte ein Individuum die Gelegenheit zu eigener Reproduktion auslassen und anstelle dessen anderen helfen, deren Brut großzuziehen? Die fakultativ sozialen allodapinen Bienen ziehen ihre Brut in offenen linearen Stengelsystemen auf (i.e. nicht in von der äußeren Umgebung abgeschirmten Brutzellen), dies erzeugt unter sozialen Insekten einzigartige Lebensgeschichten und haben sie über die vergangenen mehr als 40 Jahre zu einem wichtigen Modellsystem gemacht.
Vergleichende evolutionäre Forschung benötigt einen gesicherten Stammbaum (einen Baum der evolutionären Geschichte, der die Abstammungslinien sichtbar macht), aus dem dann die Entwicklung eines spezifischen Charakteristikums hergeleitet werden kann. Anfängliche Versuche, die Phylogenie der Allodapinen aufzulösen waren problematisch, vor allem da unabhängige auf Eigenschaften der Larven, Puppen oder Adulten beruhende Studien zu widersprüchlichen Ergebnissen geführt hatten. Analysen von DNA Sequenzen unterstützen eine sehr unterschiedliche Phylogenie, die zu einer Umordnung der Beziehungen zwischen den Gattungen führt. Das hauptsächliche Ergebnis ist, dass alle Gattungen sozial sind. Es widerspricht damit früheren Interpretationen, nach denen die soziale Evolution innerhalb der noch bestehenden Linien eingesetzt hat. Die Sozialität entwickelte sich eindeutig vor den heute lebenden Arten des Stammes zurück.
Anhand von baltischen Bernsteinfossilien eines ausgestorbenen Geschwisterstammes haben die Untersucher die Zeiträume der Entstehung dieser Bienen und ihrer Auseinanderentwicklung sowie des Bestehens ihrer sozialen Organisation einzuschätzen versucht. Nach diesen Analysen ist der Tribus vor etwa 39–80 Millionen Jahren entstanden, obwohl diese Schätzungen zurückhaltend sind und die tatsächliche Entstehungszeit vermutlich etwas früher war. Dies legt nahe, dass die Art des in dem Tribus gezeigten Sozialverhaltens keineswegs primitiv ist. Soziales Verhalten tritt in allen größeren phylogenetischen Abzweigungen auf und ist mit einer hochgradig zur weiblichen Seite neigenden Geschlechtszuweisung verbunden — eine von der Verwandtenselektion, der dominanten Theorie der letzten 30 Jahre zur Entstehung altruistischen Verhaltens nahegelegten Schlüsselgröße. Eine datierte Phylogenie ermöglicht darüber hinaus eine Untersuchung der biogeographischen Theorie. Die hier zusammengefassten Ergebnisse ergeben überraschende Einsichten über die Verbreitungsfähigkeit dieser Bienen als auch über die Rolle der nun überfluteten Landmassen im biotischen Austausch zwischen Madagaskar, Antarktis, und zuletzt auch Australien.
Similar content being viewed by others
References
Aenmey T., Tierney S.M., Pillay N., Schwarz M.P. (2006) Nesting biology of an African allodapine bee Braunsapis vitrea: female biased sex allocation in the absence of worker-like behavioural castes, Ethol. Ecol. Evol. 18, 205–220.
Aviles L. (1997) Causes and consequences of cooperation and permanent-sociality in spiders, in: Choe J., Crespi B. (Eds.), Evolution of Social Behaviour in Insects and Arachnids, Cambridge University Press, Cambridge, pp. 476–498.
Aviles L., McCormack J., Cutter A., Bukowski T. (2000) Precise highly female-biased sex ratios in a social spider, Proc. R. Soc. Lond. B. 267, 1445–1449.
Bull N.J., Schwarz M.P. (2001) Brood insurance via protogyny: a source of female biased sex allocation, Proc. R. Soc. Lond. B Biol. Sci. 268, 1869–1874.
Bull N.J., Schwarz M.P., Cooper S.J.B. (2003) Phylogenetic divergence of the Australian allodapine bees, Mol. Phylogenet. Evol. 27, 212–222.
Carpenter J.M., Strassmann J.E., Turillazzi S., Hughes C.R., Solís C.R., Cervo R. (1993) Phylogenetic relationships among paper wasp social parasites and their hosts (Hymenoptera: Vespidae; Polistinae), Cladistics 9, 129–146.
Chenoweth L.B., Tierney S.M., Smith J.A., Cooper S.J.B., Schwarz M.P. (in press) Social Complexity in bees is not sufficient to explain lack of reversions to solitary living over long time scales, BMC Evol. Biol. 7, 246.
Chenoweth L.B., Schwarz M.P. (2007) Social Biology of two Madagascan Halterapis: Evidence that Eusociality is Plesiomorphic for an Ancient Allodapine Lineage, Ann. Entomol. Soc. Am. 100, 311–319.
Choudhary M., Strassmann J.E., Queller D.C., Turillazzi S., Cervo R. (1994) Social parasites in polistine wasps are monophyletic: Implications for sympatric speciation, Proc. R. Soc. Lond. B 257, 31–35.
Cowan D.P. (1991) The solitary and presocial Vespidae, in: Ross K.G., Matthews R.W. (Eds.), The Social Biology of Wasps, Cornell University Press, New York, pp. 33–73.
Cronin A.L., Schwarz M.P. (2001) Latitudinal variation in the sociality of allodapine bees: sex ratios, relatedness and reproductive differentiation, Aust. J. Zool. 49, 1–16.
Danforth B.N., Eickwort G.C. (1997) Evolution of social behavior in the augochlorine sweat bees (Hymenoptera: Halictidae) based on a phylogenetic analysis of the genera, in: Choe J.C., Crespi B.J. (Eds.), The Evolution of Social Behaviour in Insects and Arachnids, Cambridge University Press, Cambridge, pp. 270–292.
Danforth B.N., Sauquet H., Packer L. (1999) Phylogeny of the bee genus Halictus (Hymenoptera: Halictidae) based on parsimony and likelihood analyses of nuclear EF-1α sequence data, Mol. Phylogenet. Evol. 13, 605–618.
Danforth B.N., Conway L., Ji S. (2003) Phylogeny of eusocial Lasioglossum reveals multiple losses of eusociality within a primitively eusocial clade of bees, Syst. Biol. 52, 23–36.
Duncan R.A. (2002) A time frame for construction of the Kerguelen Plateau and Broken Ridge, J. Petrology 43, 1109–1119.
Emery C. (1909) Über der Ursprung der dulotischen, parasitischen und myrmekophilen Ameisen, Biol. Centralbl. 29, 352–362.
Frey F.A., Coffin M.F., Wallace P.J., Weis D., Zhao X., Wise S.W. et al. (2000) Origin and evolution of a submarine large igneous province: the Kerguelen Plateau and Broken Ridge, southern Indian Ocean, Earth Planetary Sci. Lett. 176, 73–89.
Fuller S., Schwarz M.P., Tierney S.M. (2005) Phylogenetics of the allodapine bee genus Braunsapis: historical biogeography and long-range dispersal over water, J. Biogeogr. 32, 2135–2144.
Hay W.W., DeConto R.M., Wold C.N., Wilson K.M., Voigt S., Schulz M., Rossby-Wold A., Dullo W.-Chr., Ronov A.B., Balukhovsky A. (1999) An alternative global Cretaceous paleogeography, in: Barrera E., Johnson C. (Eds.), The Evolution of Cretaceous Ocean/Climate Systems, Geological Society of America Special Paper 332.
Herre E.A. (1985) Sex ratio adjustment in fig wasps, Science 228, 896–898.
Hogendoorn K., Watiniasih N.L., Schwarz M.P. (2001) Extended alloparental care in the almost solitary bee Exoneurella eremophila, Behav. Ecol. Sociobiol. 50, 275–282.
Hurst P.S. (2002) Social biology of Exoneurella tridentata, an Australian allodapine bee with morphological castes and perennial colonies, Flinders University, PhD Thesis, Adelaide.
Joyce N., Schwarz M.P. (2006) Sociality in the Australian allodapine bee Brevineura elongata: small colony sizes despite large benefits to group living, J. Insect Behav. 19, 45–61.
Krause D.W., Prasad G.V.R, von Koenigswald W., Sahni A., Grine F.E. (1997) Cosmopolitanism among Gondwanan Late Cretaceous mammals, Nature 390, 504–507.
Li Z.X., Powell C.McA. (2001) An outline of palaeogeographic evolution of the Australian region since the beginning of the Neoproterozoic Earth, Earth Sci. Rev. 53, 237–277.
Lin N., Michener C.D. (1972) Evolution of sociality in insects, Q. Rev. Biol. 47, 131–159.
McLoughlin S. (2001) The breakup history of Gondwana and its impact on pre-Cenozoic floristic provincialism, Aust. J. Bot. 49, 271–300.
Maeta Y., Sakagami S.F., Michener C.D. (1992) Laboratory studies on the behavior and colony structure of Braunsapis hewitti, a xylocopine bee from Taiwan (Hymenoptera: Anthophoridae), Kans. Univ. Sci. Bull. 54, 289–333.
Matthews R.W. (1991) Evolution of social behavior in sphecid wasps, in: Ross K.G., Matthews R.W. (Eds.), The Social Biology of Wasps, Cornell University Press, New York, pp. 570–602.
Michener C.D. (1965) A classification of the bees of the Australian and South Pacific regions, Bull. Am. Mus. Nat. Hist. 130, 1–362.
Michener C.D. (1970a) Nest sites of stem and twig inhabiting African bees, J. Entomol. Soc. S. Afr. 33, 1–22.
Michener C.D. (1970b) Social parasites among African allodapine bees (Hymenoptera, Anthophoridae, Ceratinini), Zool. J. Linn. Soc. 49, 199–215.
Michener C.D. (1971) Biologies of African allodapine bees, Bull. Am. Mus. Nat. Hist. 145, 219–302.
Michener C.D. (1973) Size and form of eggs of allodapine bees, J. Entomol. Soc. S. Afr. 36, 281–285.
Michener C.D. (1974) The Social Behavior of the Bees, Harvard University Press, Cambridge.
Michener C.D. (1975a) A taxonomic study of African allodapine bees, Bull. Am. Mus. Nat. Hist. 155, 67–240.
Michener C.D. (1975b) Larvae of African allodapine bees. 1. The genus Allodape, J. Entomol. Soc. S. Afr. 38, 1–12.
Michener C.D. (1975c) Larvae of African allodapine bees. 2. Braunsapis and Nasutapis, J. Entomol. Soc. S. Afr. 38, 223–242.
Michener C.D. (1975d) Larvae of African allodapine bees. 3. The genera Allodapula and Eucondylops, J. Entomol. Soc. S. Afr. 38, 243–250.
Michener C.D. (1976) Larvae of African allodapine bees. 4. Halterapis, Compsomelissa, Macrogalea, and a key to African genera, J. Entomol. Soc. S. Afr. 39, 33–37.
Michener C.D. (1977) Discordant evolution and the classification of allodapine bees, Syst. Zool. 26, 32–56.
Michener C.D. (1985) From solitary to eusocial: need there be a series of intervening species? in: Höldobler B., Lindauer M. (Eds.), Experimental behavioral ecology and sociobiology, Gustav Fischer Verlag, Stuttgart, pp. 293–305.
Michener C.D. (2000) Bees of the world, Johns Hopkins University Press, Baltimore.
Müller H. (1872) Anwendung der Darwinischen Lehre auf Bienen, Verh. Natur. Ver. Preuss. Rheinl. U. Westf. 6, 1.
Noonan B.P., Chippendale P.T. (2006) Vicariant origin of Madagascan Reptiles supports late Cretaceous Antarctic land bridge, Am. Nat. 168, 730–741.
Pauly A., Brooks R.W., Nilsson A., Pesenko Y.A., Eardley C.D., Terzo M., Griswold T., Schwarz M., Patiny S., Munzinger J., Barbier Y. (2001) Hymenoptera Apoidea de Madagascar et des îles voisines, Ann. Sci. Zool. 286, 1–390.
Popov V.B. (1945) Parazitizm pchelinykh ego osobennosti i evolyutsiya, Zhurnal Obshchei 6, 183–203.
Rage J.-C. (2003) Relationships of the Madagascan fauna during the Late Cretaceous: Northerne or Southern routes? Acta Palaeontologica Polonica 48, 661–662.
Raven P.H., Axelrod D.I. (1972) Plate tectonics and Australasian paleobiogeography, Science 176, 1379–1386.
Reeves C., de Wit M. (2000) Making ends meet in Gondwana: retracing the transforms of the Indian Ocean and reconnecting continental shear zones, Terra Nova 12, 272–280.
Reyes S.G. (1998) A cladistic analysis of the bee tribe Allodapini (Hymenoptera: Apidae: Xylocopinae), Philipp. Entomol. 12, 55–84.
Reyes S.G., Michener C.D. (1992) The genus Halterapis Michener (1969) in Madagascar, Trop. Zool. 5, 249–253.
Sampson S.D., Witmer L.M., Forster C.A., Krause D.W., O’Connor P.M., Dodson P., Ravoavy F. (1998) Predatory dinosaur remains from Madagascar: implications for the Cretaceous biogeography of Gondwana, Science 280, 1048–1051.
Sanmartín I., Ronquist F. (2004) Southern Hemisphere biogeography inferred by event-based models: plant versus animal patterns, Syst. Biol. 53, 216–243.
Savolainen R., Vepsäläinen K. (2003) Sympatric speciation through intraspecific social parasitism, Proc. Natl. Acad. Sci. USA 100, 7169–7174.
Schwarz M.P., Silberbauer L.X., Hurst P.S. (1997) Intrinsic and extrinsic factors associated with social evolution in allodapine bees, in: Choe J.C., Crespi B.J. (Eds.), The Evolution of Social Behaviour in Insects and Arachnids, Cambridge University Press, Cambridge, pp. 333–346.
Schwarz M.P., Bull N.J., Hogendoorn K. (1998) Evolution of sociality in the allodapine bees: a review of sex allocation, ecology and evolution, Insectes Soc. 45, 349–368.
Schwarz M.P., Bull N.J., Cooper S.J.B. (2003) The molecular phylogenetics of allodapine bees, with implications for the evolution of sociality and progressive rearing, Syst. Biol. 52, 1–14
Schwarz M.P., Tierney S.M., Zammit J., Schwarz P.M., Fuller S. (2005) Brood provisioning and colony composition of a Madagascan species of Halterapis: implications for social evolution in the allodapine bees, Ann. Entomol. Soc. Am. 98, 126–133
Schwarz M.P., Fuller S., Tierney S.M., Cooper S.J.B. (2006) Molecular phylogenetics of the exoneurine allodapine bees reveal an ancient and puzzling dispersal from Africa to Australia, Syst. Biol. 55, 31–45.
Schwarz M.P., Richards M.H., Danforth B.N. (2007) Changing paradigms in insect social evolution: new insights from halictine and allodapine bees, Annu. Rev. Entomol. 52, 127–150.
Sereno P.C., Wilson J.A., Conrad J.L. (2004) New dinosaurs link southern land mass in the Mid-Cretaceous, Proc. R. Soc. Lond. B, 271, 1325–1330.
Smith A.G., Smith D.G., Funneil B.M. (1994) Atlas of Mesozoic and Cenozoic coastlines, Cambridge University Press, Cambridge.
Smith J.A. (2007) Facultative social parasitism in the allodapine bee Macrogalea berentyensis, Insect Sci. 14, 65–69.
Smith J.A., Schwarz M.P. (2006) Sociality in a Madagascan allodapine bee, Macrogalea antanosy, and the impacts of the facultative social parasite, Macrogalea maizina, Insectes Soc. 53, 101–107.
Smith J.A., Tierney S.M., Park Y.C., Fuller S., Schwarz M.P. (2007) Origins of social parasitism: The importance of divergence ages in phylogenetic studies, Mol. Phylogen. Evol. (in press).
Sparks J.S., Smith W.L. (2004) Phytogeny and biogeography of the Madagascan and Australasian rainbowfishes (Teleostei: Melanotaenioidei): Gondwanan vicariance and evolution in freshwater, Mol. Phylogenet. Evol. 33, 719–734.
Sparks J.S., Smith W.L. (2005) Freshwater Fishes, Dispersal Ability, and Nonevidence: “Gondwana Life Rafts” to the Rescue, Syst. Biol. 54, 158–165.
Sumner S., Aanen D.K., Delabie J., Boomsma J.J. (2004) The evolution of social parasitism in Acromyrmex leaf-cutting ants: A test of Emery’s rule, Insectes Soc. 51, 37–42.
Thompson S., Schwarz M.P. (2006) Cooperative nesting and complex female-biased sex allocation in a tropical allodapine bee, Biol. J. Linn. Soc. 89, 355–364.
Tierney S.M. (2004) The evolution of African allodapine bees, Flinders University, PhD Thesis, Adelaide.
Tierney S.M., Schwarz M.P., Adams M. (1997) Social behaviour in an Australian allodapine bee Exoneura (Brevineura) xanthoclypeata, Aust. J. Zool. 45, 385–398.
Tierney S.M., Cronin A.L., Loussert N., Schwarz M.P. (2000) The biology of Brevineura froggatti and phylogenetic conservatism in Australian allodapine bees, Insectes Soc. 47, 96–97.
Tierney S.M., Schwarz M.P., Neville T., Schwarz P.M. (2002) Sociality in the phylogenetically basal allodapine bee genus Macrogalea (Apidae: Xylocopinae): implications for social evolution in the tribe Allodapini, Biol. J. Linn. Soc. 76, 211–224.
Vari R.P. (1992) Redescription of Mesopristes elongatus (Guichenot, 1866), an endemic Malagasy fish species (Pisces, Terapontidae), Am. Mus. Novit. 3039, 1–7.
Ward P.S. (1996) A new workerless social parasite in the ant genus Pseudomyrmex (Hymenoptera: Formicidae), with a discussion of the origin of social parasitism in ants, Syst. Entomol. 21, 253–263.
Wcislo W.T. (1996) Parasitism rates in relation to nest site in bees and wasps, J. Insect Behav. 9, 643–656.
Wcislo W.T., Danforth B.N. (1997) Secondarily solitary: the evolutionary loss of social behavior, Trends Ecol. Evol. 12, 468–474.
Wcislo W.T., Engel M.S. (1996) Social behavior and nest architecture of nomiine bees (Hymenoptera: Halictidae; Nomiinae), J. Kans. Entomol. Soc. 69 (Suppl.), 158–167.
Wcislo W.T., Tierney S.M. (in press) Evolution of communal behavior in bees and wasps: an alternative to eusociality, in: Gadau J., Fewel J. (Eds.), Organization of Insect Societies: from genomes to socio-complexity, Harvard University Press, Massachusetts.
West S.A., Herre E.A. (1998) Stabilizing selection and variance in fig wasp sex ratios, Evolution 52, 475–485.
West-Eberhard M.J. (1978) Polygyny and the evolution of social behavior in wasps, J. Kans. Entomol. Soc. 51, 832–856.
Wilson E.O. (1971) The Insect Societies, Harvard University Press, Massachusetts.
Woodburne M.O., Case J.A. (1996) Dispersal, vicariance, and the late Cretaceous to early Tertiary land mammal biogeography from South America to Australia, J. Mammal. Evol. 3, 121–161.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript editor: Eduardo A.B. Almeida
Rights and permissions
About this article
Cite this article
Tierney, S.M., Smith, J.A., Chenoweth, L. et al. Phylogenetics of allodapine bees: a review of social evolution, parasitism and biogeography. Apidologie 39, 3–15 (2008). https://doi.org/10.1051/apido:2007045
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1051/apido:2007045