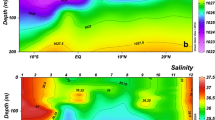
The growth and dynamics of plankton in the ocean vary with natural cycles, global climate change and the long-term evolution of ecosystems. The ocean is a large reservoir for CO2 and the food webs in the upper ocean play critical roles in regulating the global carbon cycle, changes in atmospheric CO2 and associated global warming. Microheterotrophs are a key component of the upper ocean food webs. Here, we report on the results of an analysis of the distribution of bacteria and related properties in the World Ocean. We found that, for the data set as a whole, there is a significant latitudinal gradient in all field-measured and computed bacterial properties, except growth rate. Gradients were, for the most part, driven by an equator-ward increase in the Southern Hemisphere. The biomass, rates of production and respiration and dissolved organic carbon concentrations were significantly higher in the Northern than the Southern hemispheres. In contrast, growth rates were the same in the two hemispheres. We conclude that the lower biomass and production in the Southern Hemisphere reflects greater top-down control by microbial grazers, which would be due to a lower abundance or activity of omnivorous zooplankton in the Southern than Northern Hemispheres. These large spatial differences in dynamics, structure and activity of the bacterial community and the microbial food web will be reflected in different patterns of carbon cycling, export and air–sea exchange of CO2 and the potential ability of the ocean to sequester carbon.
Similar content being viewed by others
REFERENCES
Antoine D., Andre J.-M. & Morel A. (1996) Oceanic primary production. 2. Estimation at global scale from satellite (coastal zone color scanner) chlorophyll. Global Biogeochemical Cycles 10: 57–69.
Antoine D. & Morel A. (1996) Oceanic primary production. 1. Adaptation of a spectral light-photosynthesis model in view of application to satellite chlorophyll observation. Global Biogeochemical Cycles. 10: 43–55.
Azam F. (1998) Microbial control of oceanic carbon flux: The plot thickens. Science 280: 694–696.
Battle M., Bender M. L., Tans P. P. et al. (2000) Global carbon sinks and their variability inferred from atmospheric O2 and delta 13C. Science 287: 2467–2470.
Carpenter S. R., Kitchell J. F. & Hodgson J. R. (1985) Cascading trophic interactions and lake productivity. Bioscience 35: 634–639.
Ducklow H. W. (1999) The bacterial component of the oceanic euphotic zone. FEMS Microbial Ecology 30: 1–10.
Ducklow H. W. & Carlson C. (1992) Oceanic bacterial productivity. Advances in Microbial Ecology 12: 113–181.
Fortier L., Le Fèvre J. & Legendre L. (1994) Export of biogenic carbon to fish and to the deep ocean: The role of large plankton microphages. Journal of Plankton Research 16: 809–839.
del Giorgio P. A. & Cole J. J. (1998) Bacterial growth efficiency in natural aquatic systems. Annual Review of Ecology and Systematics 29: 503–541.
Hansen J. E., Sato M., Lacis A., Ruedy R., Tegen I. & Matthews A. (1998) Climate forcings in the industrial era. Proceedings of the National Academy of Science USA 95: 12 753–12 758.
Jumars P. A., Penry D. L., Baross J. A., Perry M. J. & Frost B. W. (1989) Closing the microbal loop: dissolved carbon pathway to heterotrophic bacteria from incomplete ingestion, digestion, and absorption in animals. Deep-Sea Research 36: 483–495.
Legendre. L. & Le Fèvre. J. (1995) Microbial food webs and the export of biogenic carbon in the oceans. Aquatic Microbial Ecology 9: 69–77.
Legendre L. & Rassoulzadegan F. (1995) Plankton and nutrient dynamics in marine waters. Ophelia 41: 153–172.
Legendre L. & Rassoulzadegan F. (1996) Food-web mediated export of biogenic carbon in oceans: Hydrodynamic control. Marine Ecology Progress Series 145: 179–193.
Longhurst A. R. (1991) Role of the marine biosphere in the global carbon cycle. Limnology and Oceanography 36: 1507–1526.
Longhurst A. R. (1998) Ecological Geography of the Sea. Academic Press, New York.
McQueen D. J., Post J. R. & Mills E. L. (1986) Trophic relationships in freshwater pelagic ecosystems. Canadian Journal of Fisheries and Aquatic Sciences 43: 11–17.
Matear R. J. & Hirst A. C. (1999) Climate change feedback on the future oceanic CO2 uptake. Tellus 51: 722–733.
Pace M. L. & Cole J. J. (1994) Comparative and experimental approaches to top-down and bottom-up regulation of bacteria. Microbial Ecology 28: 181–193.
Pace M. L. & Funke E. (1991) Regulation of planktonic microbial communities by nutrients and herbivores. Ecology 72: 904–914.
Platt T. & Sathandrayath S. (1995) Scales, patterns and processes in marine ecosystems. In: Aquatic Ecology. (ed. P. S. Giller, A. G. Hildrew & D. G. Rafaelli) pp. 593–599. Blackwell Science, Oxford.
Rassoulzadegan F. & Sheldon R. W. (1986) Predator–prey interaction of a flagelate-ciliate-copepod food chain with some observations relevant to linear biomass hypothesis. Limnology and Oceanography 31: 184–188.
Redfield A. C., Ketchum B. H. & Richards F. A. (1963) The influence of organisms on the composition of seawater. In: The Sea. V. 2. (ed. M. N. Hill) pp. 26–77. Wiley-Interscience, New York.
Rivkin R. B. & Legendre L. (2001) Biogenic carbon cycling in the upper ocean: Effects of microbial respiration. Science 291: 2398–2400.
Roman M. R., Ducklow H. W. & Fuhrman J. A. et al. (1988) Production, consumption, and nutrient cycling in a laboratory mesocosm. Marine Ecology Progress Series 42: 39–52.
Sarmiento J. L., Monfray P., Maier-Reimer E., Aumount O., Murmane R. J. & Orr J. C. (2000) Sea-air CO2 fluxes and carbon transport: A comparison of three ocean general circulation models. Global Biogeochemical Cycles 14: 1267–1281.
Sarmiento J. L., Hughes T. M. C., Stoufler R. J. & Manabe S. (1998) Simulated response of ocean carbon cycle to anthropogenic climate warming. Nature 393: 245–249.
Sarmiento J. L., Le Quere C. & Pacala S. W. (1995) Limiting future atmospheric carbon dioxide. Global Biogeochemical Cycles 9: 121–137.
Schimel D., Alves D., Enting I.et al. (1996) Radiative forcing of climate change. In: 1995, International Panel on Climate Change. (ed. J. T. Houghton, L. G. Meira Filho, B. A. Callander, N. Harris, A. Kattenberg & K. Maskell) pp. 487–516. Cambridge University Press, Cambridge.
Schimel D., Enting I. G., Heimann M.et al. (1995) CO2 and the carbon cycle, in climate change. In: 1994, Intergovernmental Panel on Climate Change. (ed. J. T. Houghton, L. G. Meira Filho, J. Bruce et al.) pp. 39–71. Cambridge University Press, Cambridge.
Schlesinger W. (1997) Biogeochemistry: An Analysis of Change. Academic Press, San Diego.
Steele J. H. (1991) Marine ecosystem dynamics: comparison of scales. Ecological Research 6: 175–183.
Steele, J. H. & Henderson, E. W. (1994) Coupling between physical and biological scales Philosophical Transactions of the Royal Society of London Series B 343: 5–9.
Takahashi T., Wanninkhof R. H., Feely R. A. R.et al. (1999) Net sea-air CO2 flux over the global oceans: An improved estimate based on the sea-air, p CO2 difference. Second International Symposium on CO2 in the Oceans- the 12th Global Environment, 18–22 January 1999; Tsukuba, Japan. Centre for Global Environmental Research, National Institute of Environmental Studies, Tsukuba, Japan, pp. 9–15.
Thingstad T. F., Hagström A. & Rassoulzadegan F. (1997) Accumulation of degradable DOC in surface waters: Is it caused by a malfunctioning microbial loop. Limnology and Oceanography 42: 398–404.
Wassmann P. (1998) Retention versus export food chains: processes controlling sinking loss from marine pelagic systems. Hydrobiologia 363: 29–57.
Wickham S. A. (1995) Trophic relations between cyclopoid copepods and ciliated protists. Complex interactions link the microbial and classic food webs. Limnology and Oceanography 40: 1178–1181.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Rivkin, R., Legendre, L. Roles of food web and heterotrophic microbial processes in upper ocean biogeochemistry: Global patterns and processes. Ecol Res 17, 151–159 (2002). https://doi.org/10.1046/j.1440-1703.2002.00475.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1046/j.1440-1703.2002.00475.x