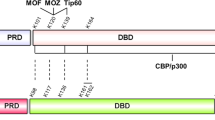
Abstract
Sam68 (Src-associated in mitosis; 68 kDa) is a member of the STAR (signal transduction and activation of RNA) family of KH domain-containing RNA binding proteins. Accumulating evidence suggests that it plays an important role in cell cycle control. Tyrosine phosphorylation by Src family kinases and breast tumor kinase can negatively regulate its RNA binding activity. To date, there are no reports of a factor, such as a phosphatase, which can positively regulate Sam68 association with RNA. Acetylation is a reversible post-translational modification known to influence the activity of DNA binding proteins. However, acetylation of a cellular RNA binding protein as a mechanism for regulating its activity has not yet been reported. Here we demonstrate Sam68 to be acetylated in vivo. A screen of several human mammary epithelial cell lines revealed variations in Sam68 acetylation. Interestingly, the highest level of acetylation was found in tumorigenic breast cancer cell lines. The screen also showed a positive correlation between Sam68 acetylation and its ability to bind RNA. The acetyltransferase CBP was shown to acetylate Sam68 and enhance its binding to poly(U) RNA. These results suggest that Sam68 association with RNA substrates may be positively regulated by acetylation, and that enhanced acetylation and RNA binding activity of Sam68 may play a role in tumor cell proliferation.








Similar content being viewed by others
References
Ait-Si-Ali S, Polesskaya A, Filleur S, Ferreira R, Duquet A, Robin P, Vervish A, Trouche D, Cabon F and Harel-Bellan A . (2000). Oncogene, 19, 2430–2437.
Arning S, Gruter P, Bilbe G and Kramer A . (1996). RNA, 2, 794–810.
Bannister AJ and Kouzarides T . (1996). Nature, 384, 641–643.
Bannister AJ, Miska EA, Gorlich D and Kouzarides T . (2000). Curr. Biol., 10, 467–470.
Barlat I, Maurier F, Duchesne M, Guitard E, Tocque B and Schweighoffer F . (1997). J. Biol. Chem., 272, 3129–3132.
Boyes J, Byfield P, Nakatani Y, Ogryzko V, Zhang W and Bieker JJ . (1998). Nature, 396, 594–598.
Chan HM, Krstic-Demonacos M, Smith L, Demonacos C and La Thangue NB . (2001). Nat. Cell Biol., 3, 667–674.
Chen T, Damaj BB, Herrera C, Lasko P and Richard S . (1997). Mol. Cell Biol., 17, 5707–5718.
Chen T and Richard S . (1998). Mol. Cell Biol., 18, 4863–4871.
Coyle JH, Guzik BW, Bor YC, Jin L, Eisner-Smerage L, Taylor SJ, Rekosh D and Hammarskjold ML . (2003). Mol. Cell Biol., 23, 92–103.
Cruz-Alvarez M and Pellicer A . (1987). J. Biol. Chem., 262, 13377–13380.
Derry JJ, Richard S, Valderrama CH, Ye X, Vasioukhin V, Cochrane AW, Chen T and Tyner AL . (2000). Mol. Cell Biol., 20, 6114–6126.
Di Fruscio M, Chen T and Richard S . (1999). Proc. Natl. Acad. Sci. USA, 96, 2710–2715.
Fumagalli S, Totty NF, Hsuan JJ and Courtneidge SA . (1994). Nature, 368, 871–874.
Grossman JS, Meyer MI, Wang YC, Mulligan GJ, Kobayashi R and Helfman DM . (1998). RNA, 4, 613–625.
Gu W and Roeder RG . (1997). Cell, 90, 595–606.
Hartmann AM, Nayler O, Schwaiger FW, Obermeier A and Stamm S . (1999). Mol. Biol. Cell, 10, 3909–3926.
Hong W, Resnick RJ, Rakowski C, Shalloway D, Taylor SJ and Blobel GA . (2002). Mol. Cancer Res., 1, 48–55.
Ishidate T, Yoshihara S, Kawasaki Y, Roy BC, Toyoshima K and Akiyama T . (1997). FEBS Lett., 409, 237–241.
Jones AR and Schedl T . (1995). Genes Dev., 9, 1491–1504.
Kiernan RE, Vanhulle C, Schiltz L, Adam E, Xiao H, Maudoux F, Calomme C, Burny A, Nakatani Y, Jeang KT, Benkirane M and Van Lint C . (1999). EMBO J., 18, 6106–6118.
Kouzarides T . (2000). EMBO J., 19, 1176–1179.
L'Hernault SW and Rosenbaum JL . (1985). Biochemistry, 24, 473–478.
Lin Q, Taylor SJ and Shalloway D . (1997). J. Biol. Chem., 272, 27274–27280.
Li QH, Haga I, Shimizu T, Itoh M, Kurosaki T and Fujisawa J . (2002). FEBS Lett, 525, 145–150.
Mandal M, Vadlamudi R, Nguyen D, Wang R-A, Costa L, Bagheri-Yarmand R, Mendelsohn J and Kumar R . (2001). J. Biol. Chem., 276, 9699–9704.
Martinez-Balbas MA, Bauer UM, Nielsen SJ, Brehm A and Kouzarides T . (2000). EMBO J., 19, 662–671.
Matter N, Herrlich P and Konig H . (2002). Nature, 420, 691–695.
Mueller F, Bommer M, Lacher U, Ruhland C, Stagge V, Adler G, Gress TM and Seufferlein T . (2003). Br. J. Cancer, 88, 699–701.
Perrotti D and Calabretta B . (2002). Oncogene, 21, 8577–8583.
Reddy TR, Suhasini M, Xu W, Yeh LY, Yang JP, Wu J, Artzt K and Wong-Staal F . (2002). J. Biol. Chem., 277, 5778–5784.
Reddy TR, Tang H, Xu W and Wong-Staal F . (2000). Oncogene, 19, 3570–3575.
Reddy TR, Xu W, Mau JK, Goodwin CD, Suhasini M, Tang H, Frimpong K, Rose DW and Wong-Staal F . (1999). Nat. Med., 5, 635–642.
Resnick RJ, Taylor SJ, Lin Q and Shalloway D . (1997). Oncogene, 15, 1247–1253.
Siomi H, Siomi MC, Nussbaum RL and Dreyfuss G . (1993). Cell, 74, 291–298.
Soros VB, Carvajal HV, Richard S and Cochrane AW . (2001). J. Virol., 75, 8203–8215.
Sterner DE and Berger SL . (2000). Microbiol. Mol. Biol. Rev., 64, 435–459.
Stoss O, Olbrich M, Hartmann AM, Konig H, Memmott J, Andreadis A and Stamm S . (2001). J. Biol. Chem., 276, 8665–8673.
Takemaru KI and Moon RT . (2000). J. Cell Biol., 149, 249–254.
Taylor SJ and Shalloway D . (1994). Nature, 368, 867–871.
Vernet C and Artzt K . (1997). Trends Genet., 13, 479–484.
Vervoorts J, Luscher-Firzlaff JM, Rottmann S, Lilischkis R, Walsemann G, Dohmann K, Austen M and Luscher B . (2003). EMBO Rep., 4, 484–490.
Vogel LB and Fujita DJ . (1995). J. Biol. Chem., 270, 2506–2511.
Wang LL, Richard S and Shaw AS . (1995). J. Biol. Chem., 270, 2010–2013.
Wu J, Zhou L, Tonissen K, Tee R and Artzt K . (1999). J. Biol. Chem., 274, 29202–29210.
Zhang W and Bieker JJ . (1998). Proc. Natl. Acad. Sci. USA, 95, 9855–9860.
Acknowledgements
We thank T Kouzarides for providing the GST-CBP (HAT domain) plasmid and R Goodman for the mouse CBP-HA cDNA. We also thank J Bjorge for critical reading of the manuscript and technical advice. This work was supported by grants to DJF from the National Cancer Institute of Canada (NCIC), the Canadian Breast Cancer Research Alliance, the Canadian Institutes of Health Research (CIHR), and the Canadian Breast Cancer Foundation (AB/NWT Chapter). IB was supported by a CIHR Doctoral Research Award and an Alberta Heritage Foundation for Medical Research (AHFMR) Studentship Award; AJ was supported by studentship awards from CIHR and AHFMR; DJF is a Scientist of the AHFMR.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Babic, I., Jakymiw, A. & Fujita, D. The RNA binding protein Sam68 is acetylated in tumor cell lines, and its acetylation correlates with enhanced RNA binding activity. Oncogene 23, 3781–3789 (2004). https://doi.org/10.1038/sj.onc.1207484
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207484
- Springer Nature Limited
Keywords
This article is cited by
-
RNA-binding proteins in ovarian cancer: a novel avenue of their roles in diagnosis and treatment
Journal of Translational Medicine (2022)
-
Control of immediate early gene expression by CPEB4-repressor complex-mediated mRNA degradation
Genome Biology (2022)
-
Sam68 promotes hepatic gluconeogenesis via CRTC2
Nature Communications (2021)
-
Sam68 contributes to intestinal inflammation in experimental and human colitis
Cellular and Molecular Life Sciences (2021)
-
Expression of Sam68 Associates with Neuronal Apoptosis and Reactive Astrocytes After Spinal Cord Injury
Cellular and Molecular Neurobiology (2017)