Abstract
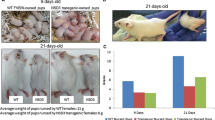
We report here for the first time, that the SV40 small t-antigen inhibits mammary gland differentiation during mid-pregnancy and that about 10% of multiparous WAP-SVt transgenic animals develop breast tumors with latencies ranging from 10–17 months. Cyclin D1 is deregulated and over expressed in the small t-antigen positive mammary gland epithelial cells (ME-cells) and in the breast tumor cells. SV40 small t-antigen immortalized ME-cells (t-ME-cells) exhibit a strong intranuclear cyclin D1 staining, also in the absence of external growth factors and the cells continue to divide for several days without serum. In addition, the expression rate of cyclin E and p21Waf1 but not of p53 is increased. Coimmunoprecipitation experiments revealed that p21Waf1 is mainly associated with the cyclin D/CDK4 but not with the cyclin E/CDK2 complex. WAP-SVT transgenic animals exhibit an almost regular mammary gland development until late pregnancy but the majority of the ME-cells are eliminated by apoptosis during the early lactation period. Tumor formation is delayed and less efficient than in T/t-antigen positive animals. Sequestration of p53 and pRb by the N-terminal truncated T-antigen molecules (T1-antigen and T2-antigen) does not affect mammary gland differentiation and the transgenic animals (WAP-SVBst-Bam) do not develop breast tumors.








Similar content being viewed by others
References
Cicala C, Avantaggiati ML, Graessmann A, Rundell K, Levine AS, Carbone M . 1994 J. Virol. 68: 3138–3144
DeCaprio JA . 1999 Biologicals 27: 23–28
Dotto GP . 2000 Biochem. Biophys. Acta 1471: M43–M56
Eul J, Graessmann M, Graessmann A . 1995 EMBO J. 14: 3226–3235
Garcia A, Cereghinie S, Sontag E . 2000 J. Biol. Chem. 31: 9385–9389
Hunter T, Pines J . 1994 Cell 79: 573–582
Kohlhoff S, Ziechmann C, Gottlob K, Graessmann M . 2000 Free Rad. Biol. Med. 29: 497–506
Li M, Lewis B, Capuco AV, Laucirica R, Furth PA . 2000 Oncogene 19: 1010–1019
Martin RG, Setlour VP, Edwards CA . 1979 J. Virol. 31: 596–607
May P, May E . 1999 Oncogene 18: 7621–7636
Mumby MC, Walter G . 1991 Cell regulation 2: 589–598
Mumby MC, Walter G . 1993 Physiol. Rev. 73: 673–699
Rundell K . 1987 J. Virol. 61: 1240–1243
Santarelli R, Tzeng YJ, Zimmermann C, Guhl E, Graessmann A . 1996 Oncogene 12: 495–505
Scheidtmann KH, Echle B, Walter G . 1982 J. Virol. 44: 116–133
Schulze-Garg C, Löhler J, Gocht A, Deppert W . 2000 Oncogene 19: 1028–1037
Senkiti S, Banerjee MR . 1979 Biochem. Biophys. Acta 582: 79–88
Siegel PM, Hardy WR, Muller WJ . 2000 BioEssays 22: 554–563
Sontag E, Sontag JM, Garcia A . 1997 EMBO J. 16: 5662–5671
Tzeng YJ, Guhl E, Graessmann M, Graessmann A . 1993 Oncogene 8: 1965–1971
Tzeng YJ, Gottlob K, Santarelli R, Graessmann A . 1996 FEBS Lett. 380: 215–218
Tzeng YJ, Zimmermann C, Guhl E, Berg B, Avantaggiati ML, Graessmann A . 1998 Oncogene 16: 2103–2114
Virshup DM . 2000 Curr. Opin. Cell Biol. 12: 180–185
Wakasugi E, Kobayashi T, Tamaki Y, Ito Y, Miyashiro I, Komoike Y, Takeda T, Shin E, Takatsuka Y, Kikkawa N, Monden T, Monden M . 1997 Am. J. Clin. Pathol. 107: 684–691
Wang S, Counterman LJ, Haslam SZ . 1990 Endocrinology 127: 2183–2189
Wang TC, Cardiff RD, Zukerberg L, Lees M, Arnold A, Schmidt EV . 1994 Nature 369: 669–671
Acknowledgements
This work was supported by the Wilhelm Sander-Stiftung (93.005.4) and Verband der Chemischen Industrie.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goetz, F., Tzeng, Y., Guhl, E. et al. The SV40 small t-antigen prevents mammary gland differentiation and induces breast cancer formation in transgenic mice; truncated large T-antigen molecules harboring the intact p53 and pRb binding region do not have this effect. Oncogene 20, 2325–2332 (2001). https://doi.org/10.1038/sj.onc.1204355
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204355
- Springer Nature Limited
Keywords
This article is cited by
-
The Relationship Between Psychosocial Stressors and Breast Cancer Biology
Current Breast Cancer Reports (2010)
-
PP2A-dependent disruption of centrosome replication and cytoskeleton organization in Drosophila by SV40 small tumor antigen
Oncogene (2008)
-
Gene expression profiling: cell cycle deregulation and aneuploidy do not cause breast cancer formation in WAP-SVT/t transgenic animals
Journal of Molecular Medicine (2005)
-
HBX causes cyclin D1 overexpression and development of breast cancer in transgenic animals that are heterozygous for p53
Oncogene (2003)
-
Knockout and transgenic mice of Trp53: what have we learned about p53 in breast cancer?
Breast Cancer Research (2002)