Abstract
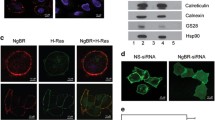
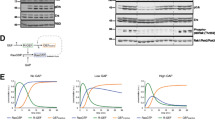
The TrkA receptor protein tyrosine kinase is involved in signalling PC12 cell differentiation and cessation of cell division in response to nerve growth factor (NGF). To assess the importance of adaptor proteins and Ras in NGF control of phosphoinositide 3-OH kinase (PI 3-kinase), specific receptor mutations in Trk have been employed. We show that phosphorylation of tyrosine 490, but not 785, of Trk is essential for activation of both Ras and PI 3-kinase in vivo, correlating with tyrosine phosphorylation of Shc and binding of Shc to the adaptor Grb2 and the Ras exchange factor Sos. A mutant receptor that lacks Y490 and Y785, but contains an introduced YxxM motif which binds the regulatory domain of PI 3-kinase, is unable to activate Ras despite causing increased PI 3-kinase activity. This indicates clearly that activation of PI 3-kinase by itself is not sufficient to cause activation of Ras, arguing against a model in which PI 3-kinase acts upstream of Ras. The Shc site of Trk is thus crucial for the activation of Ras and PI 3-kinase.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hallberg, B., Ashcroft, M., Loeb, D. et al. Nerve growth factor induced stimulation of Ras requires Trk interaction with Shc but does not involve phosphoinositide 3-OH kinase. Oncogene 17, 691–697 (1998). https://doi.org/10.1038/sj.onc.1201980
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1201980
- Springer Nature Limited
Keywords
This article is cited by
-
Splicing is an alternate oncogenic pathway activation mechanism in glioma
Nature Communications (2022)
-
Anaplastic lymphoma kinase activates the small GTPase Rap1 via the Rap1-specific GEF C3G in both neuroblastoma and PC12 cells
Oncogene (2010)
-
c-abl is involved in the association of p53 and trk A
Oncogene (2000)
-
Acidic substitution of the activation loop tyrosines in TrkA supports nerve growth factor-independent cell survival and neuronal differentiation
Oncogene (2000)
-
The ETV6-NTRK3 gene fusion encodes a chimeric protein tyrosine kinase that transforms NIH3T3 cells
Oncogene (2000)