Abstract
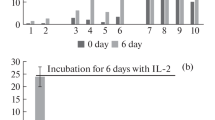
Activation of cytotoxic T cells without MHC restriction was attempted by expressing single-chain antibodies (scFv) against CD3 on the surface of tumor cells. A chimeric protein consisting of a scFv of mAb 145.2C11, the hinge-CH2-CH3 region of human IgG1, and the transmembrane and cytosolic domains of murine CD80 formed disulfide-linked dimers on the plasma membrane of cells and specifically bound lymphocytes. Anti-CD3 scFv dimers expressed on the cell surface induced CD25 (IL-2 receptor α-chain) expression and proliferation of splenocytes. CT26 tumor cells engineered to express surface scFv dimers (CT26/2C11) also induced potent lymphocyte cytotoxicity with or without addition of exogenous IL-2. Splenocytes activated by CT26/2C11 cells also killed wild-type CT26 cells, indicating that activated splenocytes could kill bystander tumor cells. Immunization of BALB/c mice with irradiated CT26/2C11 cells did not protect against a lethal challenge of CT26 cells, suggesting that systemic immunity was not induced. However, the growth of CT26 tumors containing 50% CT26/2C11 cells was significantly retarded compared with CT26 tumors, whereas CT26/2C11 tumors did not grow in syngeneic mice. These results suggest that expression of anti-CD3 scFv dimers on tumors may form the basis for a novel therapeutic strategy for tumors that exhibit defects in antigen processing or presentation.









Similar content being viewed by others
References
Urban JL, Schreiber H . Tumor antigens Annu Rev Immunol 1992 10: 617–644
Wang RF . Tumor antigens discovery: perspectives for cancer therapy Mol Med 1997 3: 716–731
Goldberg AL, Rock KL . Proteolysis, proteasomes and antigen presentation Nature 1992 357: 375–379
Maeurer MJ et al. Tumor escape from immune recognition: loss of HLA-A2 melanoma cell surface expression is associated with a complex rearrangement of the short arm of chromosome 6 Clin Cancer Res 1996 2: 641–652
Blades RA et al. Loss of HLA class I expression in prostate cancer: implications for immunotherapy Urology 1995 46: 681–686
Korkolopoulou P et al. Loss of antigen-presenting molecules (MHC class I and TAP-1) in lung cancer Br J Cancer 1996 73: 148–153
Nouri AM, Hussain RF, Oliver RT . The frequency of major histocompatibility complex antigen abnormalities in urological tumours and their correction by gene transfection or cytokine stimulation Cancer Gene Ther 1994 1: 119–123
Luboldt HJ, Kubens BS, Rubben H, Grosse Wilde H . Selective loss of human leukocyte antigen class I allele expression in advanced renal cell carcinoma Cancer Res 1996 56: 826–830
Kaklamanis L et al. Loss of major histocompatibility complex-encoded transporter associated with antigen presentation (TAP) in colorectal cancer Am J Pathol 1994 145: 505–509
Vitale M et al. HLA class I antigen and transporter associated with antigen processing (TAP1 and TAP2) down-regulation in high-grade primary breast carcinoma lesions Cancer Res 1998 58: 737–742
Restifo NP et al. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy J Natl Cancer Inst 1996 88: 100–108
Moritz D, Wels W, Mattern J, Groner B . Cytotoxic T lymphocytes with a grafted recognition specificity for ERBB2-expressing tumor cells Proc Natl Acad Sci USA 1994 91: 4318–4322
Altenschmidt U, Moritz D, Groner B . Specific cytotoxic T lymphocytes in gene therapy J Mol Med 1997 75: 259–266
Altenschmidt U, Klundt E, Groner B . Adoptive transfer of in vitro-targeted, activated T lymphocytes results in total tumor regression J Immunol 1997 159: 5509–5515
Alvarez Vallina L, Agha Mohammadi S, Hawkins RE, Russell SJ . Pharmacological control of antigen responsiveness in genetically modified T lymphocytes J Immunol 1997 159: 5889–5895
Bolhuis RL, Sturm E, Braakman E . T cell targeting in cancer therapy Cancer Immunol Immunother 1991 34: 1–8
Tibben JG et al. Pharmacokinetics, biodistribution and biological effects of intravenously administered bispecific monoclonal antibody OC/TR F(ab′)2 in ovarian carcinoma patients Int J Cancer 1996 66: 477–483
Weiner LM, Clark JI, Ring DB, Alpaugh RK . Clinical development of 2B1, a bispecific murine monoclonal antibody targeting c-erbB-2 and Fc gamma RIII J Hematother 1995 4: 453–456
Chou WC et al. Expression of chimeric monomer and dimer proteins on the plasma membrane of mammalian cells Biotechnol Bioeng 1999 65: 160–169
Reddy P, Caras I, Krieger M . Effects of O-linked glycosylation on the cell surface expression and stability of decay-accelerating factor, a glycophospholipid-anchored membrane protein J Biol Chem 1989 264: 17329–17336
Kozarsky K, Kingsley D, Krieger M . Use of a mutant cell line to study the kinetics and function of O-linked glycosylation of low density lipoprotein receptors Proc Natl Acad Sci USA 1988 85: 4335–4339
Freeman GJ et al. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7 J Exp Med 1991 174: 625–631
Minami Y, Kono T, Miyazaki T, Taniguchi T . The IL-2 receptor complex: its structure, function, and target genes Annu Rev Immunol 1993 11: 245–268
Shahinian A et al. Differential T cell costimulatory requirements in CD28-deficient mice Science 1993 261: 609–612
Van Seventer GA, Shimizu Y, Horgan KJ, Shaw S . The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells J Immunol 1990 144: 4579–4586
von Leoprechting A et al. Stimulation of CD40 on immunogenic human malignant melanomas augments their cytotoxic T lymphocyte-mediated lysis and induces apoptosis Cancer Res 1999 59: 1287–1294
Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A . T lymphocyte costimulation mediated by reorganization of membrane microdomains Science 1999 283: 680–682
Goldstein JS et al. Purified MHC class I and peptide complexes activate naive CD8+ T cells independently of the CD28/B7 and LFA-1/ICAM-1 costimulatory interactions J Immunol 1998 160: 3180–3187
Viola A, Lanzavecchia A . T cell activation determined by T cell receptor number and tunable thresholds Science 1996 273: 104–106
Harding CV, Unanue ER . Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T cell stimulation Nature 1990 346: 574–576
Kroesen BJ et al. Approaches to lung cancer treatment using the CD3 × EGP-2-directed bispecific monoclonal antibody BIS-1 Cancer Immunol Immunother 1997 45: 203–206
Bolhuis RL et al. Adoptive immunotherapy of ovarian carcinoma with bs-MAb-targeted lymphocytes: a multicenter study Int J Cancer 1992 7: (Suppl) 78–81
Kroesen BJ et al. Phase I study of intravenously applied bispecific antibody in renal cell cancer patients receiving subcutaneous interleukin 2 Br J Cancer 1994 70: 652–661
Blank Voorthuis CJ et al. Clustered CD3/TCR complexes do not transduce activation signals after bispecific monoclonal antibody-triggered lysis by cytotoxic T lymphocytes via CD3 J Immunol 1993 151: 2904–2914
Wang RF et al. Recognition of an antigenic peptide derived from tyrosinase-related protein-2 by CTL in the context of HLA-A31 and -A33 J Immunol 1998 160: 890–897
Jantzer P, Schendel DJ . Human renal cell carcinoma antigen-specific CTLs: antigen-driven selection and long-term persistence in vivo Cancer Res 1998 58: 3078–3086
Winberg G et al. Surface expression of CD28 single chain Fv for costimulation by tumor cells Immunol Rev 1996 153: 209–223
Hayden MS et al. Costimulation by CD28 sFv expressed on the tumor cell surface or as a soluble bispecific molecule targeted to the L6 carcinoma antigen Tissue Ant 1996 48: 242–254
Maloney DG et al. Monoclonal anti-idiotype antibodies against the murine B cell lymphoma 38C13: characterization and use as probes for the biology of the tumor in vivo and in vitro Hybridoma 1985 4: 191–209
Pear WS, Nolan GP, Scott ML, Baltimore D . Production of high-titer helper-free retroviruses by transient transfection Proc Natl Acad Sci USA 1993 90: 8392–8396
Belnap LP et al. Immunogenicity of chemically induced murine colon cancers Cancer Res 1979 39: 1174–1179
Solar I, Gershoni JM . Linker modification introduces useful molecular instability in a single chain antibody Protein Eng 1995 8: 717–723
Acknowledgements
This study was supported by grants from the National Science Council, Taipei, Taiwan (NSC 88–2318-B001–013-M51) and Academia Sinica.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liao, KW., Lo, YC. & Roffler, S. Activation of lymphocytes by anti-CD3 single-chain antibody dimers expressed on the plasma membrane of tumor cells. Gene Ther 7, 339–347 (2000). https://doi.org/10.1038/sj.gt.3301080
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301080
- Springer Nature Limited
Keywords
This article is cited by
-
Facile repurposing of peptide–MHC-restricted antibodies for cancer immunotherapy
Nature Biotechnology (2023)
-
A recombinant scFv-FasLext as a targeting cytotoxic agent against human Jurkat-Ras cancer
Journal of Biomedical Science (2013)
-
Evaluating combinations of costimulatory antibody–ligand fusion proteins for targeted cancer immunotherapy
Cancer Immunology, Immunotherapy (2013)
-
Adenovirus-mediated intratumoral expression of immunostimulatory proteins in combination with systemic Treg inactivation induces tumor-destructive immune responses in mouse models
Cancer Gene Therapy (2011)
-
Gene expression imaging by enzymatic catalysis of a fluorescent probe via membrane-anchored β-glucuronidase
Gene Therapy (2007)