Abstract
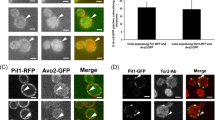
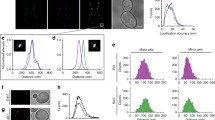
Plasma membranes are organized into domains of different protein and lipid composition. Eisosomes are key complexes for yeast plasma membrane organization, containing primarily Pil1 and Lsp1. Here we show that both proteins consist mostly of a banana-shaped BAR domain common to membrane sculpting proteins, most similar to the ones of amphiphysin, arfaptin 2 and endophilin 2. Our data reveal a previously unrecognized family of BAR-domain proteins involved in plasma membrane organization.



Similar content being viewed by others
References
Simons, K. & Ikonen, E. Nature 387, 569–572 (1997).
Lingwood, D. & Simons, K. Science 327, 46–50 (2010).
Walther, T.C. et al. Nature 439, 998–1003 (2006).
Grossmann, G., Opekarova, M., Malinsky, J., Weig-Meckl, I. & Tanner, W. EMBO J. 26, 1–8 (2007).
Strádalová, V. et al. J. Cell Sci. 122, 2887–2894 (2009).
Fröhlich, F. et al. J. Cell Biol. 185, 1227–1242 (2009).
Minor, W., Cymborowski, M., Otwinowski, Z. & Chruszcz, M. Acta Crystallogr. D Biol. Crystallogr. 62, 859–866 (2006).
Peter, B.J. et al. Science 303, 495–499 (2004).
Tarricone, C. et al. Nature 411, 215–219 (2001).
Masuda, M. & Mochizuki, N. Semin. Cell Dev. Biol. 21, 391–398 (2010).
Acknowledgements
We would like to thank P. de Camilli, E. Conti, F. Förster, T. Keil, W. Minor, S. Schuck, S. Suppmann, A. Wlodawer and the MPI-B Crystallization Facility for discussion and help with experiments and the German Research Foundation (N.E.Z. and T.C.W.), Academy of Finland (grant 130750, J.T.H.) and Boehringer Ingelheim fellowships (L.K.) for funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to design and execution of experiments. N.E.Z. produced the protein, grew crystals, solved the structure of Lsp1 and performed the confocal microscopy. N.E.Z. and T.C.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Tables 1–2 and Supplementary Methods (PDF 1412 kb)
Supplementary Video 1
Pil1-GFP R126E in the pil1Δ lsp1Δ strain forms long rods traversing the cytoplasm. Ylr413w-RFPmars is used as a membrane staining marker. z-stack images collected at 0.2-μm distances. (AVI 88 kb)
Rights and permissions
About this article
Cite this article
Ziółkowska, N., Karotki, L., Rehman, M. et al. Eisosome-driven plasma membrane organization is mediated by BAR domains. Nat Struct Mol Biol 18, 854–856 (2011). https://doi.org/10.1038/nsmb.2080
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2080
- Springer Nature America, Inc.
This article is cited by
-
A Review of Mechanics-Based Mesoscopic Membrane Remodeling Methods: Capturing Both the Physics and the Chemical Diversity
The Journal of Membrane Biology (2022)
-
Functional diversity of complex I subunits in Candida albicans mitochondria
Current Genetics (2016)
-
Plasma membrane organization promotes virulence of the human fungal pathogen Candida albicans
Journal of Microbiology (2016)
-
Insights into eisosome assembly and organization
Journal of Biosciences (2012)
-
Eisosomes and plasma membrane organization
Molecular Genetics and Genomics (2012)