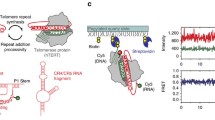
Abstract
Telomerase shows repeat-addition processivity (RAP): synthesis of multiple telomeric DNA repeats without primer dissociation. Leu14 mutants in the telomerase essential N-terminal domain of Tetrahymena thermophila telomerase reverse transcriptase retain full activity and anchor-site function but lose RAP, suggesting models for how this domain facilitates DNA translocation.



Similar content being viewed by others
References
Lingner, J. et al. Science 276, 561–567 (1997).
Greider, C.W. Mol. Cell. Biol. 11, 4572–4580 (1991).
Hammond, P.W., Lively, T.N. & Cech, T.R. Mol. Cell. Biol. 17, 296–308 (1997).
Lue, N.F. & Li, Z. Nucleic Acids Res. 35, 5213–5222 (2007).
Jacobs, S.A., Podell, E.R. & Cech, T.R. Nat. Struct. Mol. Biol. 13, 218–225 (2006).
Romi, E. et al. Proc. Natl. Acad. Sci. USA 104, 8791–8796 (2007).
Moriarty, T.J., Marie-Egyptienne, D.T. & Autexier, C. Mol. Cell. Biol. 24, 3720–3733 (2004).
Finger, S.N. & Bryan, T.M. Nucleic Acids Res. 36, 1260–1272 (2008).
Wyatt, H.D., Lobb, D.A. & Beattie, T.L. Mol. Cell. Biol. 27, 3226–3240 (2007).
Miller, M.C., Liu, J.K. & Collins, K. EMBO J. 19, 4412–4422 (2000).
Bar-Nahum, G. et al. Cell 120, 183–193 (2005).
Lai, C.K., Miller, M.C. & Collins, K. Mol. Cell 11, 1673–1683 (2003).
Mason, D.X., Goneska, E. & Greider, C.W. Mol. Cell. Biol. 23, 5606–5613 (2003).
Acknowledgements
We thank K. Collins, N. Lue and A. Berman for useful discussions.
Author information
Authors and Affiliations
Contributions
A.J.Z. made the initial discovery and designed and carried out experiments; E.R.P. contributed to data analysis and interpretation; T.R.C. contributed to experimental design and models.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Methods and Supplementary Discussion (PDF 1581 kb)
Supplementary Movie
Leu14 (shown as the branched amino acid) acts as a latch between the TEN domain and the remainder of telomerase, opening and closing during the reaction cycle. See Figure 3 in the main text for details. (MOV 9167 kb)
Rights and permissions
About this article
Cite this article
Zaug, A., Podell, E. & Cech, T. Mutation in TERT separates processivity from anchor-site function. Nat Struct Mol Biol 15, 870–872 (2008). https://doi.org/10.1038/nsmb.1462
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1462
- Springer Nature America, Inc.
This article is cited by
-
Giardia telomeres and telomerase
Parasitology Research (2024)
-
Therapeutic Targets in Telomerase and Telomere Biology of Cancers
Indian Journal of Clinical Biochemistry (2020)
-
Structural basis of template-boundary definition in Tetrahymena telomerase
Nature Structural & Molecular Biology (2015)
-
The RNA accordion model for template positioning by telomerase RNA during telomeric DNA synthesis
Nature Structural & Molecular Biology (2011)
-
Structural basis for telomerase catalytic subunit TERT binding to RNA template and telomeric DNA
Nature Structural & Molecular Biology (2010)