Key Points
-
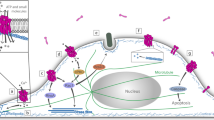
The Rho GTPases are frequently targeted by many different bacterial cytotoxins. Although it might seem surprising that a divergent array of toxins that have different modes of action and come from a range of bacterial species target the same effector, the Rho GTPases have been identified as eukaryotic master 'molecular switches' in many signal transduction pathways and, crucially, in regulation of the actin cytoskeleton.
-
Rho GTPases have many regulatory roles in host–pathogen interactions and host defence, including maintaining epithelial barriers, leukocyte migration, phagocytosis, T cell–APC (antigen presenting cell) interaction, and nuclear factor κB (NF-κB) regulation.
-
The GTPase cycle is the key to regulating the activity of the Rho GTPases. Bacterial cytotoxins can affect all the phases of the GTPase cycle.
-
The irreversible inhibition of Rho GTPases by cytotoxins is achieved through covalent modification by glucosylation (for example, by the clostridial glucosylating cytotoxins), ADP-ribosylation (the C3-like exoenzymes) or proteolytic cleavage (Yersinia enterocolitica YopT).
-
Reversible inhibition is achieved by bacterial effectors with a GTPase-activating protein-like activity (for example Yersinia spp. YopE, Salmonella SptP and Pseudomonas aeruginosa ExoS and ExoT).
-
GTPases can also be activated by deamidation and transglutamination by Escherichia coli and Yersinia pseudotuberculosis cytotoxic necrotizing factors and Bordetella spp. dermotoxic necrotizing toxin, respectively.
-
Additionally, some toxins are able to modify actin directly, including the family of binary actin-ADP-ribosylating toxins, the actin ADP-ribosyltransferase SpvB from Salmonella, and the actin crosslinking repeats-in-toxin cytotoxin from Vibrio cholerae.
Abstract
Many bacterial cytotoxins act on eukaryotic cells by targeting the regulators that are involved in controlling the cytoskeleton or by directly modifying actin, with members of the Rho GTPase family being particularly important targets. The actin cytoskeleton, and especially the GTPase 'molecular switches' that are involved in its control, have crucial functions in innate and adaptive immunity, and have pivotal roles in the biology of infection. In this review, we briefly discuss the role of the actin cytoskeleton and the Rho GTPases in host–pathogen interactions, and review the mode of actions of bacterial protein toxins that target these components.





Similar content being viewed by others
References
Wennerberg, K. & Der, C. J. Rho-family GTPases: it's not only Rac and Rho (and I like it). J. Cell Sci. 117, 1301–1312 (2004).
Ridley, A. J. & Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389–399 (1992). An early indication that Rho regulates the formation of stress fibres, whereas Cdc42 and Rac regulate the formation of filopodia and lamellipodia, respectively.
Ridley, A. J., Paterson, H. F., Johnston, C. L., Diekmann, D. & Hall, A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410 (1992).
Nobes, C. D. & Hall, A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62 (1995).
Burridge, K. & Wennerberg, K. Rho and Rac take center stage. Cell 116, 167–179 (2004).
Raftopoulou, M. & Hall, A. Cell migration: Rho GTPases lead the way. Dev. Biol. 265, 23–32 (2004).
Van Aelst, L. & D'Souza-Schorey, C. Rho GTPases and signaling networks. Genes Dev. 11, 2295–2322 (1997).
Svitkina, T. M. & Borisy, G. G. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 145, 1009–1026 (1999).
Millard, T. H., Sharp, S. J. & Machesky, L. M. Signalling to actin assembly via the WASP (Wiskott–Aldrich syndrome protein)-family proteins and the Arp2/3 complex. Biochem. J. 380, 1–17 (2004).
Welch, M. D. & Mullins, R. D. Cellular control of actin nucleation. Annu. Rev. Cell Dev. Biol. 18, 247–288 (2002).
Rohatgi, R. et al. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–231 (1999). Provided an early indication that WASP is the protein that links cell signalling to actin polymerization.
Miki, H., Suetsugu, S. & Takenawa, T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 17, 6932–6941 (1998).
Eden, S., Rohatgi, R., Podtelejnikov, A. V., Mann, M. & Kirschner, M. W. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature 418, 790–793 (2002).
Stradal, T. E. et al. Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 14, 303–311 (2004).
Braga, V. M., Machesky, L. M., Hall, A. & Hotchin, N. A. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell–cell contacts. J. Cell Biol. 137, 1421–1431 (1997).
Nusrat, A. et al. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc. Natl Acad. Sci. USA 92, 10629–10633 (1995).
Bruewer, M., Hopkins, A. M., Hobert, M. E., Nusrat, A. & Madara, J. L. RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am. J. Physiol. Cell Physiol. 287, C327–C335 (2004).
Vaezi, A., Bauer, C., Vasioukhin, V. & Fuchs, E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev. Cell 3, 367–381 (2002).
Fenteany, G., Janmey, P. A. & Stossel, T. P. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr. Biol. 10, 831–838 (2000).
Nobes, C. D. & Hall, A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 144, 1235–1244 (1999).
Snapp, K. R., Heitzig, C. E. & Kansas, G. S. Attachment of the PSGL-1 cytoplasmic domain to the actin cytoskeleton is essential for leukocyte rolling on P-selectin. Blood 99, 4494–4502 (2002).
Ivetic, A. & Ridley, A. J. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology 112, 165–176 (2004).
Setiadi, H. & McEver, R. P. Signal-dependent distribution of cell surface P-selectin in clathrin-coated pits affects leukocyte rolling under flow. J. Cell Biol. 163, 1385–1395 (2003).
Brakebusch, C. & Fassler, R. The integrin–actin connection, an eternal love affair. EMBO J. 22, 2324–2333 (2003).
Worthylake, R. A. & Burridge, K. RhoA and ROCK promote migration by limiting membrane protrusions. J. Biol. Chem. 278, 13578–13584 (2003).
Smith, A., Bracke, M., Leitinger, B., Porter, J. C. & Hogg, N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J. Cell Sci. 116, 3123–3133 (2003).
Worthylake, R. A., Lemoine, S., Watson, J. M. & Burridge, K. RhoA is required for monocyte tail retraction during transendothelial migration. J. Cell Biol. 154, 147–160 (2001).
van Wetering, W. S. et al. VCAM-1-mediated Rac signaling controls endothelial cell–cell contacts and leukocyte transmigration. Am. J. Physiol. Cell Physiol. 285, C343–C352 (2003).
Caron, E. & Hall, A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282, 1717–1721 (1998). Showed the role of Rho GTPases in two independent phagocytic mechanisms.
Vicente-Manzanares, M. & Sanchez-Madrid, F. Role of the cytoskeleton during leukocyte responses. Nature Rev. Immunol. 4, 110–122 (2004).
May, R. C., Caron, E., Hall, A. & Machesky, L. M. Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nature Cell Biol. 2, 246–248 (2000).
Olazabal, I. M. et al. Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcγR, phagocytosis. Curr. Biol. 12, 1413–1418 (2002).
Bokoch, G. M. & Diebold, B. A. Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood 100, 2692–2696 (2002).
Roberts, A. W. et al. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity 10, 183–196 (1999).
Dreikhausen, U. et al. Regulation by rho family GTPases of IL-1 receptor induced signaling: C3-like chimeric toxin and Clostridium difficile toxin B inhibit signaling pathways involved in IL-2 gene expression. Eur. J. Immmunol. 31, 1610–1619 (2001).
Hao, S., Kurosaki, T. & August, A. Differential regulation of NFAT and SRF by the B cell receptor via a PLCγCa(2+)-dependent pathway. EMBO J. 22, 4166–4177 (2003).
Croker, B. A. et al. The Rac2 guanosine triphosphatase regulates B lymphocyte antigen receptor responses and chemotaxis and is required for establishment of B-1a and marginal zone B lymphocytes. J. Immunol. 168, 3376–3386 (2002).
Miletic, A. V., Swat, M., Fujikawa, K. & Swat, W. Cytoskeletal remodeling in lymphocyte activation. Curr. Opin. Immunol. 15, 261–268 (2003).
Acuto, O. & Cantrell, D. T cell activation and the cytoskeleton. Annu. Rev. Immunol. 18, 165–184 (2000). A review that describes the relationships between the actin cytoskeleton and host cell immunology.
Stowers, L., Yelon, D., Berg, L. J. & Chant, J. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc. Natl Acad. Sci. USA 92, 5027–5031 (1995).
Costello, P. S. et al. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-κB pathways. Proc. Natl Acad. Sci. USA 96, 3035–3040 (1999).
Benvenuti, F. et al. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science 305, 1150–1153 (2004).
Barois, N., Forquet, F. & Davoust, J. Actin microfilaments control the MHC class II antigen presentation pathway in B cells. J. Cell Sci. 111, 1791–1800 (1998).
Schraufstatter, I. U., Trieu, K., Sikora, L., Sriramarao, P. & DiScipio, R. Complement c3a and c5a induce different signal transduction cascades in endothelial cells. J. Immunol. 169, 2102–2110 (2002).
Ghosh, S., May, M. J. & Kopp, E. B. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16, 225–260 (1998).
Fiorentini, C., Falzano, L., Travaglione, S. & Fabbri, A. Hijacking Rho GTPases by protein toxins and apoptosis: molecular strategies of pathogenic bacteria. Cell Death Differ. 10, 147–152 (2003).
Karin, M. & Ben-Neriah, Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18, 621–663 (2000).
Perona, R. et al. Activation of the nuclear factor-κB by Rho, Cdc42, and Rac-1 proteins. Genes Dev. 11, 463–475 (1997).
Fritz, G. & Kaina, B. Ras-related GTPase Rhob represses NF-κB signaling. J. Biol. Chem. 276, 3115–3122 (2001).
Arbibe, L. et al. Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nature Immunol. 1, 533–540 (2000).
Teusch, N., Lombardo, E., Eddleston, J. & Knaus, U. G. The low molecular weight GTPase RhoA and atypical protein kinase ζ are required for TLR2-mediated gene transcription. J. Immunol. 173, 507–514 (2004).
Bartlett, J. G., Moon, N., Chang, T. W., Taylor, N. & Onderdonk, A. B. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology 75, 778–782 (1978).
Kelly, C. P., Pothoulakis, C. & LaMont, J. T. Clostridium difficile colitis. N. Engl. J. Med. 330, 257–262 (1994).
Busch, C. & Aktories, K. Microbial toxins and the glucosylation of Rho family GTPases. Curr. Opin. Struct. Biol. 10, 528–535 (2000).
Von Eichel-Streiber, C., Boquet, P., Sauerborn, M. & Thelestam, M. Large clostridial cytotoxins — a family of glycosyltransferases modifying small GTP-binding proteins. Trends Microbiol. 4, 375–382 (1996).
Hofmann, F., Busch, C., Prepens, U., Just, I. & Aktories, K. Localization of the glucosyltransferase activity of Clostridium difficile toxin B to the N-terminal part of the holotoxin. J. Biol. Chem. 272, 11074–11078 (1997).
Just, I. et al. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J. Biol. Chem. 270, 13932–13936 (1995).
Just, I. et al. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375, 500–503 (1995). Describes the mode of action of C. difficile toxin B in glucosylating Rho.
Herrmann, C., Ahmadian, M. R., Hofmann, F. & Just, I. Functional consequences of monoglucosylation of H-Ras at effector domain amino acid threonine-35. J. Biol. Chem. 273, 16134–16139 (1998).
Sehr, P. et al. Glucosylation and ADP-ribosylation of Rho proteins — Effects on nucleotide binding, GTPase activity, and effector-coupling. Biochemistry 37, 5296–5304 (1998).
Genth, H., Aktories, K. & Just, I. Monoglucosylation of RhoA at Threonine-37 blocks cytosol–membrane cycling. J. Biol. Chem. 274, 29050–29056 (1999).
Ottlinger, M. E. & Lin, S. Clostridium difficile toxin B induces reorganization of actin, vinculin, and talin in cultures cells. Exp. Cell Res. 174, 215–229 (1988).
Fiorentini, C. & Thelestam, M. Clostridium difficile toxin A and its effects on cells. Toxicon. 29, 543–567 (1991).
Prepens, U., Just, I., Von Eichel-Streiber, C. & Aktories, K. Inhibition of FcεRI-mediated activation of rat basophilic leukemia cells by Clostridium difficile toxin B (monoglucosyltransferase). J. Biol. Chem. 271, 7324–7329 (1996).
Schmidt, M. et al. Inhibition of receptor signaling to phospholipase D by Clostridium difficile toxin B — role of Rho proteins. J. Biol. Chem. 271, 2422–2426 (1996).
Brito, G. A. C. et al. Mechanism of Clostridium difficile toxin A-induced apoptosis in T84 cells. J. Infect. Dis. 186, 1438–1447 (2002).
Hippenstiel, S. et al. Rho protein inactivation induced apoptosis of cultured human endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 283, L830–838 (2002).
Servant, G. et al. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287, 1037–1040 (2000).
Hippenstiel, S. et al. Glucosylation of small GTP-binding Rho proteins disrupts endothelial barrier function. Am. J. Physiol. 272, L38–L43 (1997).
Nusrat, A. et al. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect. Immun. 69, 1329–1336 (2001).
Riegler, M. et al. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J. Clin. Invest. 95, 2004–2011 (1995).
Gerhard, R., Schmidt, G., Hofmann, F. & Aktories, K. Activation of Rho GTPases by Escherichia coli cytotoxic necrotizing factor 1 increases intestinal permeability in Caco-2 cells. Infect. Immun. 66, 5125–5131 (1998).
Hecht, G., Pothoulakis, C., LaMont, J. T. & Madara, J. L. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J. Clin. Invest. 82, 1516–1524 (1988).
Jou, T. -S., Schneeberger, E. E. & Nelson, W. J. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J. Cell Biol. 142, 101–115 (1998).
Pothoulakis, C. et al. Clostridium difficile toxin A stimulates intracellular calcium release and chemotactic response in human granulocytes. J. Clin. Invest. 81, 1741–1745 (1988).
Warny, M. et al. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J. Clin. Invest. 105, 1147–1156 (2000).
Savidge, T. C. et al. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology 125, 413–420 (2003).
Jefferson, K. K., Smith, M. F. Jr & Bobak, D. A. Roles of intracellular calcium and NF-κB in the Clostridium difficile toxin A-induced up-regulation and secretion of IL-8 from human monocytes. J. Immunol. 163, 5183–5191 (1999).
Ishida, Y. et al. Essential involvement of IFN-γ in Clostridium difficile toxin A-induced enteritis. J. Immunol. 172, 3018–3025 (2004).
Pothoulakis, C. & LaMont, J. T. Microbes and microbial toxins: paradigms for microbial–mucosal interactions. II. The integrated response of the intestine to Clostridium difficile toxins. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G178–G183 (2001).
Pothoulakis, C. et al. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc. Natl Acad. Sci. USA 91, 947–951 (1994).
Anton, P. M. et al. Corticotropin-releasing hormone (CRH) requirement in Clostridium difficile toxin A-mediated intestinal inflammation. Proc. Natl Acad. Sci. USA 101, 8503–8508 (2004).
Wershil, B. K., Castagliuolo, I. & Pothoulakis, C. Direct evidence of mast cell involvement in Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology 114, 956–964 (1998).
Huang, S., Chen, L. Y., Zuraw, B. L., Ye, R. D. & Pan, Z. K. Chemoattractant-stimulated NF-κB activation is dependent on the low molecular weight GTPase RhoA. J. Biol. Chem. 276, 40977–40981 (2001).
Chen, M. L., Pothoulakis, C. & LaMont, J. T. Protein kinase C signaling regulates ZO-1 translocation and increased paracellular flux of T84 colonocytes exposed to Clostridium difficile toxin A. J. Biol. Chem. 277, 4247–4254 (2002).
He, D. et al. Clostridium difficile toxin A causes early damage to mitochondria in cultured cells. Gastroenterology 119, 139–150 (2000).
Hippenstiel, S. et al. Rho proteins and the p38-MAPK pathway are important mediators for LPS-induced interleukin-8 expression in human endothelial cells. Blood 95, 3044–3051 (2000).
Gerhard, R. et al. Clostridium difficile toxin A induces expression of the stress-induced early gene product RhoB. J. Biol. Chem. 280, 1499–1505 (2005).
Aktories, K., Braun, U., Rösener, S., Just, I. & Hall, A. The rho gene product expressed in E. coli is a substrate of botulinum ADP-ribosyltransferase C3. Biochem. Biophys. Res. Commun. 158, 209–213 (1989).
Chardin, P. et al. The mammalian G protein rho C is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilament in Vero cells. EMBO J. 8, 1087–1092 (1989). Shows that Rho is the target of ADP-ribosylation by C. botulinum exoenzyme C3.
Just, I. et al. Purification and characterization of an ADP-ribosyltransferase produced by Clostridium limosum. J. Biol. Chem. 267, 10274–10280 (1992).
Wilde, C., Vogelsgesang, M. & Aktories, K. Rho-specific Bacillus cereus ADP-ribosyltransferase C3cer cloning and characterization. Biochemistry 42, 9694–9702 (2003).
Wilde, C., Chhatwal, G. S., Schmalzing, G., Aktories, K. & Just, I. A novel C3-like ADP-ribosyltransferase from Staphylococcus aureus modifying RhoE and Rnd3. J. Biol. Chem. 276, 9537–9542 (2001).
Sekine, A., Fujiwara, M. & Narumiya, S. Asparagine residue in the rho gene product is the modification site for botulinum ADP-ribosyltransferase. J. Biol. Chem. 264, 8602–8605 (1989).
Genth, H., Schmidt, M., Gerhard, R., Aktories, K. & Just, I. Activation of phospholipase D1 by ADP-ribosylated RhoA. Biochem. Biophys. Res. Commun. 302, 127–132 (2003).
Genth, H. et al. Entrapment of Rho ADP-ribosylated by Clostridium botulinum C3 exoenzyme in the Rho–guanine nucleotide dissociation inhibitor-1 complex. J. Biol. Chem. 278, 28523–28527 (2003).
Paterson, H. F. et al. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J. Cell Biol. 111, 1001–1007 (1990). Gives the functional consequences of ADP-ribosylation of RhoA and demonstrates how to use C3 exoenzyme as a tool.
Madden, J. C., Ruiz, N. & Caparon, M. Cytolysin-mediated translocation (CMT): a functional equivalent type III secretion in Gram-positive bacteria. Cell 104, 143–152 (2001).
Bricker, A. L., Cywes, C., Ashbaugh, C. D. & Wessels, M. R. NAD+-glycohydrolase acts as an intracellular toxin to enhance the extracellular survival of group A streptococci. Mol. Microbiol. 44, 257–269 (2002).
Haque, A. et al. Production, purification, and characterization of botulinolysin, a thiol-activated hemolysin of Clostridium botulinum. Infect. Immun. 60, 71–78 (1992).
Beecher, D. J., Olsen, T. W., Somers, E. B. & Wong, A. C. Evidence for contribution of tripartite hemolysin BL, phosphatidylcholine-preferring phospholipase C, and collagenase to virulence of Bacillus cereus endophthalmitis. Infect. Immun. 68, 5269–5276 (2000).
Wilde, C., Chhatwal, G. S. & Aktories, K. C3stau, a new member of the family of C3-like ADP-ribosyltransferases. Trends Microbiol. 10, 5–7 (2002).
Iriarte, M. & Cornelis, G. R. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol. Microbiol. 29, 915–929 (1998).
Shao, F., Merritt, P. M., Bao, Z., Innes, R. W. & Dixon, J. E. A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell 109, 575–588 (2002). First to show that YopT was a cysteine protease.
Shao, F. et al. Biochemical characterization of the Yersinia YopT protease: cleavage site and recognition elements in Rho GTPases. Proc. Natl Acad. Sci. USA 100, 904–909 (2003).
Zumbihl, R. et al. The cytotoxin YopT of Yersinia enterocolitica induces modification and cellular redistribution of the small GTP-binding protein RhoA. J. Biol. Chem. 274, 29289–29293 (1999).
Aepfelbacher, M. et al. Characterization of YopT effects on Rho GTPases in Yersinia enterocolitica infected cells. J. Biol. Chem. 278, 33217–33223 (2003).
Grosdent, N., Maridonneau-Parini, I., Sory, M. -P. & Cornelis, G. Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagosytosis. Infect. Immun. 70, 4165–4176 (2002).
Guan, K. L. & Dixon, J. E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science 249, 553–556 (1990). Identified the phosphatase activity of YopH.
Persson, C., Carballeira, N., Wolf-Watz, H. & Fällman, M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 16, 2307–2318 (1997).
Black, D. S., Marie-Cardine, A., Schraven, B. & Bliska, J. B. The Yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signalling complex in macrophages. Cell. Microbiol. 2, 401–414 (2000).
Barz, C., Abahji, T. N., Trülzsch, K. & Heesemann, J. The Yersinia Ser/Thr protein kinase YpkA/YopO directly interacts with the small GTPases RhoA and Rac-1. FEBS Lett. 482, 139–143 (2000).
Goehring, U. -M., Schmidt, G., Pederson, K. J., Aktories, K. & Barbieri, J. T. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J. Biol. Chem. 274, 36369–36372 (1999).
von Pawel-Rammingen, U. et al. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36, 737–748 (2000).
Fu, Y. & Galan, J. E. A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature 401, 293–297 (1999). First to determine that bacterial toxins can be molecular mimics of Rho GAPs.
Kaniga, K., Uralil, J., Bliska, J. B. & Galandrini, R. A secreted protein tyrosine phosphatase with modular effector domains in the bacterial pathogen Salmonella typhimurium. Mol. Microbiol. 21, 633–641 (1996).
Krall, R., Schmidt, G., Aktories, K. & Barbieri, J. T. Pseudomonas aeruginosa ExoT is a Rho GTPase-activating protein. Infect. Immun. 68, 6066–6068 (2000).
Caprioli, A., Falbo, V., Roda, L. G., Ruggeri, F. M. & Zona, C. Partial purification and characterization of an Escherichia coli toxic factor that induces morphological cell alterations. Infect. Immun. 39, 1300–1306 (1983).
Caprioli, A. et al. A cell division-active protein from E. coli. Biochem. Biophys. Res. Commun. 118, 587–593 (1984).
Oswald, E. et al. Cytotoxic necrotizing factor type 2 produced by virulent Escherichia coli modifies the small GTP-binding proteins Rho involved in assembly of actin stress fibers. Proc. Natl Acad. Sci. USA 91, 3814–3818 (1994).
Falzano, L. et al. Induction of phagocytic behaviour in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol. Microbiol. 9, 1247–1254 (1993).
Fiorentini, C. et al. Escherichia coli cytotoxic necrotizing factor 1 (CNF1), a toxin that activates the Rho GTPase. J. Biol. Chem. 272, 19532–19537 (1997).
Schmidt, G. et al. Gln63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor 1. Nature 387, 725–729 (1997).
Flatau, G. et al. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 387, 729–733 (1997). Identified the CNF toxins as deaminases.
de Rycke, J. et al. Evidence for two types of cytotoxic necrotizing factor in human and animal clinical isolates of Escherichia coli. J. Clin. Microbiol. 28, 694–699 (1990).
Lockman, H. A., Gillespie, R. A., Baker, B. D. & Shakhnovich, E. Yersinia pseudotuberculosis produces a cytotoxic necrotizing factor. Infect. Immun. 70, 2708–2714 (2002).
Lemichez, E., Flatau, G., Bruzzone, M., Boquet, P. & Gauthier, M. Molecular localization of the Escherichia coli cytotoxic necrotizing factor CNF1 cell-binding and catalytic domains. Mol. Microbiol. 24, 1061–1070 (1997).
Buetow, L., Flatau, G., Chiu, K., Boquet, P. & Ghosh, P. Structure of the Rho-activating domain of Escherichia coli cytotoxic necrotizing factor 1. Nature Struct. Biol. 8, 584–588 (2001).
Lerm, M., Schmidt, G., Goehring, U. -M., Schirmer, J. & Aktories, K. Identification of the region of Rho involved in substrate recognition by Escherichia coli cytotoxic necrotizing factor 1 (CNF1). J. Biol. Chem. 274, 28999–29004 (1999).
Hoffmann, C. et al. The Yersinia pseudotuberculosis cytotoxic necrotizing factor (CNFY) selectively activates RhoA. J. Biol. Chem. 279, 16026–16032 (2004).
Lerm, M. et al. Deamidation of Cdc42 and Rac by Escherichia coli cytotoxic necrotizing factor 1 (CNF1): activation of c-Jun-N-terminal kinase in HeLa cells. Infect. Immun. 67, 496–503 (1999).
Lerm, M., Pop, M., Fritz, G., Aktories, K. & Schmidt, G. Proteasomal degradation of cytotoxic necrotizing factor 1-activated Rac. Infect. Immun. 70, 4053–4058 (2002).
Doye, A. et al. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell 111, 553–564 (2002).
Rippere-Lampe, K. E., O'Brien, A. D., Conran, R. & Lockman, H. A. Mutation of the gene encoding cytotoxic necrotizing factor type 1 (cnf1) attenuates the virulence of uropathogenic Escherichia coli. Infect. Immun. 69, 3954–3964 (2001).
Rippere-Lampe, K. E. et al. Cytotoxic necrotizing factor type 1-positive Escherichia coli causes increased inflammation and tissue damage to the prostate in a rat prostatitis model. Infect. Immun. 69, 6515–6519 (2001).
Khan, N. A. et al. Cytotoxic necrotizing factor-1 contributes to Escherichia coli K1 invasion of the central nervous system. J. Biol. Chem. 277, 15607–15612 (2002).
Hopkins, A. M., Walsh, S. V., Verkade, P., Boquet, P. & Nusrat, A. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J. Cell Sci. 116, 725–742 (2003).
Horiguchi, Y. et al. Bordetella bronchiseptica dermonecrotizing toxin induces reorganization of actin stress fibers through deamidation of Gln-63 of the GTP-binding protein Rho. Proc. Natl Acad. Sci. USA 94, 11623–11626 (1997).
Masuda, M. et al. Activation of Rho through a cross-link with polyamines catalyzed by Bordetella dermonecrotizing toxin. EMBO J. 19, 521–530 (2000).
Aktories, K. et al. Botulinum C2 toxin ADP-ribosylates actin. Nature 322, 390–392 (1986). First to show that C2 toxin directly ADP-ribosylates actin.
Ohishi, I., Iwasaki, M. & Sakaguchi, G. Purification and characterization of two components of botulinum C2 toxin. Infect. Immun. 30, 668–673 (1980).
Vandekerckhove, J., Schering, B., Bärmann, M. & Aktories, K. Botulinum C2 toxin ADP-ribosylates cytoplasmic β/γ-actin in arginine 177. J. Biol. Chem. 263, 696–700 (1988).
Wegner, A. & Aktories, K. ADP-ribosylated actin caps the barbed ends of actin filaments. J. Biol. Chem. 263, 13739–13742 (1988).
Wille, M., Just, I., Wegner, A. & Aktories, K. ADP-ribosylation of the gelsolin–actin complex by clostridial toxins. J. Biol. Chem. 267, 50–55 (1992).
Mauss, S., Chaponnier, C., Just, I., Aktories, K. & Gabbiani, G. ADP-ribosylation of actin isoforms by Clostridium botulinum C2 toxin and Clostridium perfringens iota toxin. Eur. J. Biochem. 194, 237–241 (1990).
Han, S., Craig, J. A., Putnam, C. D., Carozzi, N. B. & Tainer, J. A. Evolution and mechanism from structures of an ADP-ribosylating toxin and NAD complex. Nature Struct. Biol. 6, 932–936 (1999).
Tsuge, H. et al. Crystal structure and site-directed mutagenesis of enzymatic components from Clostridium perfringens iota-toxin. J. Mol. Biol. 325, 471–483 (2003).
Reuner, K. H., Presek, P., Boschek, C. B. & Aktories, K. Botulinum C2 toxin ADP-ribosylates actin and disorganizes the microfilament network in intact cells. Eur. J. Cell Biol. 43, 134–140 (1987).
Ohishi, I. Response of mouse intestinal loop to botulinum C2 toxin: enterotoxic activity induced by cooperation of nonlinked protein components. Infect. Immun. 40, 691–695 (1983).
Ohishi, I. & Odagiri, Y. Histopathological effect of Botulinum C2 toxin on mouse intestines. Infect. Immun. 43, 54–58 (1984).
Considine, R. V. & Simpson, L. L. Cellular and molecular actions of binary toxins possessing ADP-ribosyltransferase activity. Toxicon. 29, 913–936 (1991).
Goncalves, C., Decre, D., Barbut, F., Burghoffer, B. & Petit, J. C. Prevalence and characterization of a binary toxin (actin-specific ADP-ribosyltransferase) from Clostridium difficile. J. Clin. Microbiol. 42, 1933–1939 (2004).
Roudier, C., Fierer, J. & Guiney, D. G. Characterization of translation termination mutations in the spv operon of the Salmonella virulence plasmid pSDL2. J. Bacteriol. 174, 6418–6423 (1992).
Lesnick, M. L., Reiner, N. E., Fierer, J. & Guiney, D. G. The Salmonella spvB virulence gene encodes an enzyme that ADP-ribosylates actin and destabilizes the cytoskeleton of eukaryotic cells. Mol. Microbiol. 39, 1464–1470 (2001).
Tezcan-Merdol, D. et al. Actin is ADP-ribosylated by the Salmonella enterica virulence-associated protein SpvB. Mol. Microbiol. 39, 606–619 (2001).
Fullner, K. J., Lencer, W. I. & Mekalanos, J. J. Vibrio cholerae-induced cellular responses of polarized T84 intestinal epithelial cells are dependent on production of cholera toxin and the RTX toxin. Infect. Immun. 69, 6310–6317 (2001).
Fullner, K. J. & Mekalanos, J. J. In vivo covalent cross-linking of cellular actin by the Vibrio cholerae RTX toxin. EMBO J. 19, 5315–5323 (2000).
Lommel, S. et al. Actin pedestal formation by enteropathogenic Escherichia coli and intracellular motility of Shigella flexneri are abolished in N-WASP-defective cells. EMBO Rep. 2, 850–857 (2001).
Frischknecht, F. & Way, M. Surfing pathogens and the lessons learned for actin polymerization. Trends Cell Biol. 11, 30–38 (2001).
Gouin, E. et al. The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nature 427, 457–461 (2004).
Suzuki, T., Miki, H., Takenawa, T. & Sasakawa, C. Neural Wiskott-Aldrich syndrome protein is implicated in the actin-based motility of Shigella flexneri. EMBO J. 17, 2767–2776 (1998).
Dacheux, D. et al. Pseudomonas aeruginosa cystic fibrosis isolates induce rapid, type III secretion-dependent, but ExoU-independent, oncosis of macrophages and polymorphonuclear neutrophils. Infect. Immun. 68, 2916–2924 (2000).
Yahr, T. L., Vallis, A. J., Hancock, M. K., Barbieri, J. T. & Frank, D. W. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl Acad. Sci. USA 95, 13899–13904 (1998).
Iglewski, B. H., Liu, P. V. & Kabat, D. Mechanism of action of Pseudomonas aeruginosa exotoxin Aiadenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect. Immun. 15, 138–144 (1977).
Maresso, A. W., Baldwin, M. R. & Barbieri, J. T. Ezrin/radixin/moesin proteins are high affinity targets for ADP-ribosylation by Pseudomonas aeruginosa ExoS. J. Biol. Chem. 279, 38402–38408 (2004).
Louvet-Vallee, S. ERM proteins: from cellular architecture to cell signaling. Biol. Cell 92, 305–316 (2000).
Liu, S., Yahr, T. L., Frank, D. W. & Barbieri, J. T. Biochemical relationships between the 53-kilodalton (Exo53) and 49-kilodalton (ExoS) forms of exoenzyme S of Pseudomonas aeruginosa. J. Bacteriol. 179, 1609–1613 (1997).
Sun, J. & Barbieri, J. T. Pseudomonas aeruginosa ExoT ADP-ribosylates CT10-regulator of kinase (Crk). J. Biol. Chem. 278, 32794–32803 (2003).
Sun, J., Maresso, A. W., Kim, J. J. & Barbieri, J. T. How bacterial ADP-ribosylating toxins recognize substrates. Nat. Struct. Mol. Biol. 11, 868–876 (2004). Provided structural predictions of how the bacterial ADP-ribosyltransferases recognize their substrates.
Feller, S. M. Crk family adaptors-signalling complex formation and biological roles. Oncogene 20, 6348–6371 (2001).
Hardt, W. -D., Chen, L. -M., Schuebel, K. E., Bustelo, X. R. & Galán, J. E. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93, 815–826 (1998).
Stender, S. et al. Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol. Microbiol. 36, 1206–1221 (2000).
Hayward, R. D. & Koronakis, V. Direct nucleation and bundling of actin by the SipC protein of invasive Salmonella. EMBO J. 18, 4926–4934 (1999).
McGhie, E. J., Hayward, R. D. & Koronakis, V. Cooperation between actin-binding proteins of invasive Salmonella: SipA potentiates SipC nucleation and bundling of actin. EMBO J. 20, 2131–2139 (2001).
McGhie, E. J., Hayward, R. D. & Koronakis, V. Control of actin turnover by a Salmonella invasion protein. Mol. Cell 13, 497–510 (2004).
Kubori, T. & Galan, J. E. Temporal regulation of Salmonella virulence effector function by proteasome-dependent protein degradation. Cell 115, 333–342 (2003).
Hernandez, L. D., Hueffer, K., Wenk, M. R. & Galan, J. E. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science 304, 1805–1807 (2004).
Hurd, T. W., Gao, L., Roh, M. H., Macara, I. G. & Margolis, B. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nature Cell Biol. 5, 137–142 (2003).
Hirase, T. et al. Regulation of tight junction permeability and occludin phosphorylation by Rhoa-p160ROCK-dependent and -independent mechanisms. J. Biol. Chem. 276, 10423–10431 (2001).
Genth, H. et al. Difference in protein substrate specificity between hemorrhagic toxin and lethal toxin from Clostridium sordellii. Biochem. Biophys. Res. Commun. 229, 370–374 (1996).
Chaves-Olarte, E. et al. A novel cytotoxin from Clostridium difficile serogroup F is a functional hybrid between two other large clostridial cytotoxins. J. Biol. Chem. 274, 11046–11052 (1999).
Popoff, M. R. et al. Ras, Rap, and Rac small GTP-binding proteins are targets for Clostridium sordellii lethal toxin glucosylation. J. Biol. Chem. 271, 10217–10224 (1996).
Selzer, J. et al. Clostridium novyi α-toxin-catalyzed incorporation of GlcNAc into Rho subfamily proteins. J. Biol. Chem. 271, 25173–25177 (1996).
Aktories, K., Wilde, C. & Vogelsgesang, M. Rho-modifying C3-like ADP-ribosyltransferases. Rev. Physiol. Biochem. Pharmacol. (2004).
Krall, R., Sun, J., Pederson, K. J. & Barbieri, J. T. In vivo rho GTPase-activating protein activity of Pseudomonas aeruginosa cytotoxin ExoS. Infect. Immun. 70, 360–367 (2002).
Welch, M. D., Rosenblatt, J., Skoble, J., Portnoy, D. A. & Mitchison, T. J. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science 281, 105–108 (1998).
Ho, H. -Y. H. et al. Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex. Cell 118, 203–216 (2004).
Acknowledgements
J.T.B. is supported by grants from National Institutes of Health. K.A. is supported by the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- FOCAL CONTACTS
-
Regions of cell attachment to the extracellular matrix. Adhesion receptors and specific cytoskeletal proteins are clustered in these regions.
- LAMELLIPODIA
-
Broad, flat protrusions at the leading edge of a moving cell that are enriched with a branched network of actin filaments. They are often associated with cell migration.
- FOCAL COMPLEXES
-
Small dot-like adhesion structures that are present mainly at the edges of the lamellipodia.
- FILOPODIA
-
Thin, transient actin protrusions that extend out from the cell surface and are formed by the elongation of bundled actin filaments.
- TIGHT JUNCTIONS
-
Regions of apical adhesion between adjacent epithelial or endothelial cells. Tight junctions regulate paracellular flux, and contribute to the maintenance of cell polarity by stopping molecules from diffusing in the plane of the membrane.
- ADHERENS JUNCTIONS
-
Epithelial cell–cell adhesion complexes associated with actin filaments that contain cadherins.
- MAST CELL
-
A type of leukocyte with large secretory granules containing histamine and various protein mediators.
Rights and permissions
About this article
Cite this article
Aktories, K., Barbieri, J. Bacterial cytotoxins: targeting eukaryotic switches. Nat Rev Microbiol 3, 397–410 (2005). https://doi.org/10.1038/nrmicro1150
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1150
- Springer Nature Limited
This article is cited by
-
Golgi stress induces SIRT2 to counteract Shigella infection via defatty-acylation
Nature Communications (2022)
-
Comparative splenic proteomic analysis of susceptible and resistant GIFT tilapia following challenge with Streptococcus agalactiae
Aquaculture International (2021)
-
Escherichia coli Rho GTPase-activating toxin CNF1 mediates NLRP3 inflammasome activation via p21-activated kinases-1/2 during bacteraemia in mice
Nature Microbiology (2021)
-
Reversible senescence of human colon cancer cells after blockage of mitosis/cytokinesis caused by the CNF1 cyclomodulin from Escherichia coli
Scientific Reports (2018)
-
A systematic analysis of the RNA-targeting potential of secreted bacterial effector proteins
Scientific Reports (2017)