Abstract
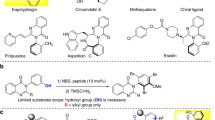
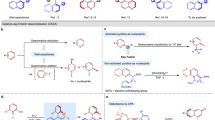
This protocol describes a method for the laboratory synthesis of enantiomerically enriched, chiral tetrahydroisoquinolines through the application of a chiral sulfinamido urea catalyst for the Povarov reaction. Tetrahydroisoquinolines are bicyclic organic frameworks present in a wide assortment of natural and synthetic biologically important compounds including martinelline, scoulerine and tubocurarine. The methodology involves the [4+2] cycloaddition of a N-arylimines with electron-rich olefins such as vinyl lactams and dihydropyrroles in the presence of a two-catalyst system consisting of an achiral strong Brønsted acid (o-nitrobenzenesulfonic acid), together with the chiral sulfinamido urea derivative 1. The anion-binding properties of the urea lead to the association of the ion pair that results from protonation of the imine substrate. Cycloaddition is followed by spontaneous proton loss with re-aromatization to provide the tetrahydroisoquinoline products in highly enantio-enriched form.








Similar content being viewed by others
References
Eigen, M. Proton transfer acid-base catalysis: enzymatic hydrolysis. Part 1. Elementary processes. Angew. Chem. Int. Ed. 3, 1–19 (1964).
Akiyama, T., Itoh, J., Yokota, K. & Fuchibe, K. Enantioselective Mannich-type reaction catalyzed by a chiral Brønsted acid. Angew. Chem. Int. Ed. 43, 1566–1568 (2004).
Uraguchi, D., Sorimachi, K. & Terada, M. Organocatalytic asymmetric aza-Friedel-Crafts alkylation of furan. J. Am. Chem. Soc. 126, 11804–11805 (2004).
Nakashima, D. & Yamamoto, H. Design of chiral N-triflyl phosphoramide as a strong chiral Brønsted acid and its application to asymmetric Diels-Alder reaction. J. Am. Chem. Soc. 128, 9626–9627 (2006).
Hatano, M., Maki, T., Moriyama, K., Arinobe, M. & Ishihara, K. Pyridinium 1,1′-binaphthyl-2,2′-disulfonates as highly effective chiral Brønsted acid-base combined salt catalysts for enantioselective Mannich-type reaction. J. Am. Chem. Soc. 130, 16858–16860 (2008).
Ishihara, K., Kaneeda, M. & Yamamoto, H. Lewis-acid assisted chiral Brønsted acid for enantioselective protonation of silyl enol ethers and ketene bis(trialkyl silyl) acetals. J. Am. Chem. Soc. 116, 11179–11180 (1994).
Yamamoto, H. & Futatsugi, K. 'Designer acids': combined acid catalysis for asymmetric synthesis. Angew. Chem. Int. Ed. 44, 1924–1942 (2005).
Raheem, I.T., Thiara, P.S., Peterson, E.A. & Jacobsen, E.N. Enantioselective Pictet-Spengler-type cyclizations of hydroxylactams: H-bond donor catalysis by anion binding. J. Am. Chem. Soc. 129, 13404–13405 (2007).
Doyle, A.G. & Jacobsen, E.N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 107, 5713–5743 (2007).
Schreiner, P.R. & Wittkopp, A. H-bonding additives act like Lewis acid catalysts. Org. Lett. 4, 217–220 (2002).
Kouznetsov, V.V. Recent synthetic developments in a powerful imino Diels-Alder reaction (Povarov reaction): application to the synthesis of N-polyheterocycles and related alkaloids. Tetrahedron 65, 2721–2750 (2009).
Xu, H., Zuend, S.J., Woll, M.G., Tao, Y. & Jacobsen, E.N. Asymmetric cooperative catalysis of strong Brønsted acid-promoted reactions using chiral ureas. Science 327, 986–990 (2010).
Gerard, B. et al. Application of a catalytic asymmetric Povarov reaction using chiral ureas to the synthesis of a tetrahydroquinoline library. ACS Combi. Sci. 14, 621–630 (2012).
Tan, K.L. & Jacobsen, E.N. Indium-mediated asymmetric allylation of acylhydrazones using a chiral urea catalyst. Angew. Chem. Int. Ed. 46, 1315–1317 (2007).
Ishitani, H. & Kobayashi, S. Catalytic asymmetric aza Diels-Alder reactions using a chiral lanthanide Lewis acid. Enantioselective synthesis of tetrahydroquinoline derivatives using a catalytic amount of a chiral source. Tetrahedron Lett. 37, 7357–7360 (1996).
Akiyama, T., Morita, H. & Fuchibe, K. Chiral Brønsted acid-catalyzed inverse electron-demand aza Diels-Alder reaction. J. Am. Chem. Soc. 128, 13070–13071 (2006).
Liu, H., Dagousset, G., Masson, G., Retailleau, P. & Zhu, J.P. Chiral Brønsted acid-catalyzed enantioselective three-component Povarov reaction. J. Am. Chem. Soc. 131, 4598–4599 (2009).
Witherup, K.M. et al. Martinelline and martinellic acid, novel G-protein linked receptor antagonists from the tropical plant Martinella iquitosensis (Bignoniaceae). J. Am. Chem. Soc. 117, 6682–6685 (1995).
Xia, C.F., Heng, L.S. & Ma, D.W. Total synthesis of (±)-martinelline. Tetrahedron Lett. 43, 9405–9409 (2002).
Batey, R.A. et al. A three-component coupling protocol for the synthesis of substituted hexahydropyrrolo[3,2-c]quinolines. Chem. Commun. 651–652 (1999).
Keinicke, L., Fristrup, P., Norrby, P.-O. & Madsen, R. Nonradical zinc-Barbier reaction for diastereoselective synthesis of vicinal amino alcohols. J. Am. Chem. Soc. 127, 15756–15761 (2005).
Kraus, G.A. & Neuenschwander, K. Facile synthesis of N-acyl-2-pyrrolines. J. Org. Chem. 46, 4791–4792 (1981).
Trost, B.M. & Marrs, C.M. A [3+2] cycloaddition and [4+3] cycloaddition approach to N-heterocycles via palladium-catalyzed TMM reactions with imines. J. Am. Chem. Soc. 115, 6636–6645 (1993).
Larrow, J.F. & Jacobsen, E.N. (R,R)-N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamino manganese(III) chloride, a highly enantioselective epoxidation catalyst. Org. Syn. 75, 1 (1998).
Acknowledgements
This work was carried out with support from the US National Institutes of Health (grants no. GM-43214 and P50 GM-69721), and by fellowship support from the Dreyfus Foundation (to H.X.).
Author information
Authors and Affiliations
Contributions
H.X. designed and performed the experiments, and co-wrote the paper. H.Z. performed the synthesis of catalyst 1. E.N.J. designed and supervised the experiments, analyzed data and co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Xu, H., Zhang, H. & Jacobsen, E. Chiral sulfinamidourea and strong Brønsted acid–cocatalyzed enantioselective Povarov reaction to access tetrahydroquinolines. Nat Protoc 9, 1860–1866 (2014). https://doi.org/10.1038/nprot.2014.125
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.125
- Springer Nature Limited
This article is cited by
-
A reaction mode of carbene-catalysed aryl aldehyde activation and induced phenol OH functionalization
Nature Communications (2017)