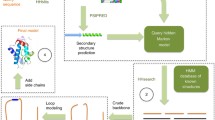
Abstract
Determining the structure and function of a novel protein is a cornerstone of many aspects of modern biology. Over the past decades, a number of computational tools for structure prediction have been developed. It is critical that the biological community is aware of such tools and is able to interpret their results in an informed way. This protocol provides a guide to interpreting the output of structure prediction servers in general and one such tool in particular, the protein homology/analogy recognition engine (Phyre). New profile–profile matching algorithms have improved structure prediction considerably in recent years. Although the performance of Phyre is typical of many structure prediction systems using such algorithms, all these systems can reliably detect up to twice as many remote homologies as standard sequence-profile searching. Phyre is widely used by the biological community, with >150 submissions per day, and provides a simple interface to results. Phyre takes 30 min to predict the structure of a 250-residue protein.




Similar content being viewed by others
References
CASP 7 special issue. Proteins 69 (Suppl. 8), 1–207 (2007).
Baker, D. & Sali, A. Protein structure prediction and structural genomics. Science 294, 93–96 (2001).
Watson, J.D., Laskowski, R.A. & Thornton, J.M. Predicting protein function from sequence and structural data. Curr. Opin. Struct. Biol. 15, 275–284 (2005).
Qian, B. et al. High-resolution structure prediction and the crystallographic phase problem. Nature 450, 259–264 (2007).
Rava, P. & Hussain, M.M. Acquisition of triacylglycerol transfer activity by microsomal triglyceride transfer protein during evolution. Biochemistry 46, 12263–12274 (2007).
Park, H. et al. Discovery of novel alpha-glucosidase inhibitors based on the virtual screening with the homology-modeled protein structure. Bioorg. Med. Chem. 16, 284–292 (2008).
Altschul, S.F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Karplus, K., Barrett, C. & Hughey, R. Hidden Markov models for detecting remote protein homologies. Bioinformatics 14, 846–856 (1998).
Ohlson, T., Wallner, B. & Elofsson, A. Profile–profile methods provide improved fold-recognition: a study of different profile–profile alignment methods. Proteins 57, 188–197 (2004).
Bennett-Lovsey, R.M., Herbert, A.D., Sternberg, M.J.E. & Kelley, L.A. Exploring the extremes of sequence/structure space with ensemble fold recognition in the program Phyre. Proteins 70, 611–625 (2008).
Murzin, A.G., Brenner, S.E., Hubbard, T. & Chothia, C. SCOP: a structural classification of proteins database for the investigation of sequences and structures. J. Mol. Biol. 247, 536–540 (1995).
Berman, H.M. et al. The protein data bank. Nucleic Acids Res. 28, 235–242 (2000).
McGuffin, L.J., Bryson, K. & Jones, D.T. The PSIPRED protein structure prediction server. Bioinformatics 16, 404–405 (2000).
Pollastri, G., Przybylski, D., Rost, B. & Baldi, P. Improving the prediction of protein secondary structure in three and eight classes using recurrent neural networks and profiles. Proteins 47, 228–235 (2002).
Cole, C., Barber, J.D. & Barton, G.J. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 36 (Web server issue): W197–W201 (2008).
Ward, J.J., McGuffin, L.J., Bryson, K., Buxton, B.F. & Jones, D.T. The DISOPRED server for the prediction of protein disorder. Bioinformatics 20, 2138–2139 (2004).
Tress, M.L., Jones, D.T. & Valenica, A. Predicting reliable regions in protein alignments from sequence profiles. J. Mol. Biol. 330, 705–718 (2003).
Marchler-Bauer, A. et al. CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res. 35 (Database issue): D237–D240 (2007).
Finn, R.D. et al. The Pfam protein families database. Nucleic Acids Res. 36 (Database issue): D281–D288 (2008).
Wass, M.N. & Sternberg, M.J.E. ConFunc—functional annotation in the twilight zone. Bioinformatics 24, 798–806 (2008).
Yao, H. et al. An accurate, sensitive, and scalable method to identify functional sites in protein structures. J. Mol. Biol. 326, 255–261 (2003).
Kinoshita, K. & Nakamura, H. Identification of protein biochemical functions by similarity search using the molecular surface database eF-site. Protein Sci. 12, 1589–1595 (2003).
Laskowski, R.A. et al. Protein clefts in molecular recognition and function. Prot. Sci. 5, 2438–2452 (1996).
Liang, J., Edelsbrunner, H., Fu, P., Sudhakar, P.V. & Subramaniam, S. Analytical shape computation of macromolecules I and II. Proteins 33, 1–17 and 18–29 (1998).
Jones, D.T. Predicting novel protein folds by using FRAGFOLD. Proteins 45 (Suppl. 5): 127–132 (2001).
Kim, D.E., Chivian, D. & Baker, D. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 32 (Web server issue): W526–W531 (2004).
Zhang, Y. Template-based modeling and free modeling by I-TASSER in CASP7. Proteins 69 (Suppl. 8): 108–117 (2007).
Hulo, N. et al. The 20 years of PROSITE. Nucleic Acids Res. 36 (Database issue): D245–D249 (2008).
Acknowledgements
L.A.K. is supported by the BBSRC grant number LDAD PO6300.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Sternberg is a director and shareholder of Equinox Pharma Ltd. And Dr. Kelley has in the past acted as a consultant for Equinox Pharma Ltd. The Phyre server and code is freely available to academics and is available to commercial users via a license from Equinox Pharma Ltd.
Rights and permissions
About this article
Cite this article
Kelley, L., Sternberg, M. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc 4, 363–371 (2009). https://doi.org/10.1038/nprot.2009.2
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.2
- Springer Nature Limited
This article is cited by
-
Glutathione peroxidase LtGPX3 contributes to oxidative stress tolerance, virulence, and plant defense suppression in the peach gummosis fungus Lasiodiplodia theobromae
Phytopathology Research (2024)
-
Isolation and characterization of novel Staphylococcus aureus bacteriophage Hesat from dairy origin
Applied Microbiology and Biotechnology (2024)
-
In silico secretome analyses of the polyphagous root-knot nematode Meloidogyne javanica: a resource for studying M. javanica secreted proteins
BMC Genomics (2023)
-
Genome-wide identification and characterization of parthenocarpic fruit set-related gene homologs in cucumber (Cucumis sativus L.)
Scientific Reports (2023)
-
Comprehensive analyses of microtubule-associated protein MAP65 family genes in Cucurbitaceae and CsaMAP65s expression profiles in cucumber
Journal of Applied Genetics (2023)