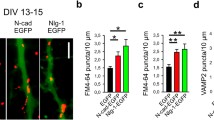
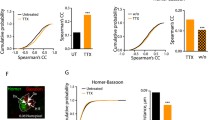
Abstract
N-cadherin is a homophilic adhesion protein that remains expressed at mature excitatory synapses beyond its developmental role in synapse formation. We investigated the trans-synaptic activity of N-cadherin in regulating synapse function in rodent cultured hippocampal neurons using optical methods and electrophysiology. Interfering with N-cadherin in postsynaptic neurons reduced basal release probability (pr) at inputs to the neuron, and this trans-synaptic impairment of release accompanied impaired vesicle endocytosis. Moreover, loss of the GluA2 AMPA-type glutamate receptor subunit, which decreased pr by itself, occluded the interference with postsynaptic N-cadherin. The loss of postsynaptic N-cadherin activity, however, did not affect the compensatory upregulation of pr induced by chronic activity silencing, whereas postsynaptic β-catenin deletion blocked this presynaptic homeostatic adaptation. Our findings suggest that postsynaptic N-cadherin helps link basal pre- and postsynaptic strengths to control the pr offset, whereas the pr gain adjustment requires a distinct trans-synaptic pathway involving β-catenin.








Similar content being viewed by others
References
Togashi, H. et al. Interneurite affinity is regulated by heterophilic nectin interactions in concert with the cadherin machinery. J. Cell Biol. 174, 141–151 (2006).
Abe, K., Chisaka, O., Van Roy, F. & Takeichi, M. Stability of dendritic spines and synaptic contacts is controlled by alpha N-catenin. Nat. Neurosci. 7, 357–363 (2004).
Bamji, S.X. et al. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron 40, 719–731 (2003).
Murase, S., Mosser, E. & Schuman, E.M. Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron 35, 91–105 (2002).
Okuda, T., Yu, L.M., Cingolani, L.A., Kemler, R. & Goda, Y. beta-Catenin regulates excitatory postsynaptic strength at hippocampal synapses. Proc. Natl. Acad. Sci. USA 104, 13479–13484 (2007).
Tang, L., Hung, C.P. & Schuman, E.M. A role for the cadherin family of cell adhesion molecules in hippocampal long-term potentiation. Neuron 20, 1165–1175 (1998).
Bozdagi, O. et al. Persistence of coordinated long-term potentiation and dendritic spine enlargement at mature hippocampal CA1 synapses requires N-cadherin. J. Neurosci. 30, 9984–9989 (2010).
Mendez, P., De Roo, M., Poglia, L., Klauser, P. & Muller, D. N-cadherin mediates plasticity-induced long-term spine stabilization. J. Cell Biol. 189, 589–600 (2010).
Saglietti, L. et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron 54, 461–477 (2007).
Jüngling, K. et al. N-cadherin transsynaptically regulates short-term plasticity at glutamatergic synapses in embryonic stem cell-derived neurons. J. Neurosci. 26, 6968–6978 (2006).
Stan, A. et al. Essential cooperation of N-cadherin and neuroligin-1 in the transsynaptic control of vesicle accumulation. Proc. Natl. Acad. Sci. USA 107, 11116–11121 (2010).
Hessler, N.A., Shirke, A.M. & Malinow, R. The probability of transmitter release at a mammalian central synapse. Nature 366, 569–572 (1993).
Murthy, V.N., Sejnowski, T.J. & Stevens, C.F. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron 18, 599–612 (1997).
Rosenmund, C., Clements, J.D. & Westbrook, G.L. Nonuniform probability of glutamate release at a hippocampal synapse. Science 262, 754–757 (1993).
Pang, Z.P. & Sudhof, T.C. Cell biology of Ca2+-triggered exocytosis. Curr. Opin. Cell Biol. 22, 496–505 (2010).
Zucker, R.S. & Regehr, W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 64, 355–405 (2002).
Branco, T., Staras, K., Darcy, K.J. & Goda, Y. Local dendritic activity sets release probability at hippocampal synapses. Neuron 59, 475–485 (2008).
Frank, C.A., Kennedy, M.J., Goold, C.P., Marek, K.W. & Davis, G.W. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron 52, 663–677 (2006).
Jakawich, S.K. et al. Local presynaptic activity gates homeostatic changes in presynaptic function driven by dendritic BDNF synthesis. Neuron 68, 1143–1158 (2010).
Lindskog, M. et al. Postsynaptic GluA1 enables acute retrograde enhancement of presynaptic function to coordinate adaptation to synaptic inactivity. Proc. Natl. Acad. Sci. USA 107, 21806–21811 (2010).
Frank, C.A., Pielage, J. & Davis, G.W. A presynaptic homeostatic signaling system composed of the Eph receptor, ephexin, Cdc42, and CaV2.1 calcium channels. Neuron 61, 556–569 (2009).
Futai, K. et al. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat. Neurosci. 10, 186–195 (2007).
Ryan, T.A., Reuter, H. & Smith, S.J. Optical detection of a quantal presynaptic membrane turnover. Nature 388, 478–482 (1997).
Tokuoka, H. & Goda, Y. Activity-dependent coordination of presynaptic release probability and postsynaptic GluR2 abundance at single synapses. Proc. Natl. Acad. Sci. USA 105, 14656–14661 (2008).
Takeichi, M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu. Rev. Biochem. 59, 237–252 (1990).
Brault, V. et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264 (2001).
Passafaro, M., Nakagawa, T., Sala, C. & Sheng, M. Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2. Nature 424, 677–681 (2003).
Ripley, B., Otto, S., Tiglio, K., Williams, M.E. & Ghosh, A. Regulation of synaptic stability by AMPA receptor reverse signaling. Proc. Natl. Acad. Sci. USA 108, 367–372 (2011).
Song, J.Y., Ichtchenko, K., Sudhof, T.C. & Brose, N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc. Natl. Acad. Sci. USA 96, 1100–1105 (1999).
Burrone, J. & Murthy, V.N. Synaptic gain control and homeostasis. Curr. Opin. Neurobiol. 13, 560–567 (2003).
Pozo, K. & Goda, Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron 66, 337–351 (2010).
Turrigiano, G.G. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135, 422–435 (2008).
Burrone, J., O'Byrne, M. & Murthy, V.N. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature 420, 414–418 (2002).
Han, E.B. & Stevens, C.F. Development regulates a switch between post- and presynaptic strengthening in response to activity deprivation. Proc. Natl. Acad. Sci. USA 106, 10817–10822 (2009).
Nakayama, K., Kiyosue, K. & Taguchi, T. Diminished neuronal activity increases neuron-neuron connectivity underlying silent synapse formation and the rapid conversion of silent to functional synapses. J. Neurosci. 25, 4040–4051 (2005).
Wierenga, C.J., Walsh, M.F. & Turrigiano, G.G. Temporal regulation of the expression locus of homeostatic plasticity. J. Neurophysiol. 96, 2127–2133 (2006).
Bredt, D.S. & Nicoll, R.A. AMPA receptor trafficking at excitatory synapses. Neuron 40, 361–379 (2003).
Newpher, T.M. & Ehlers, M.D. Glutamate receptor dynamics in dendritic microdomains. Neuron 58, 472–497 (2008).
Sheng, M. & Hoogenraad, C.C. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu. Rev. Biochem. 76, 823–847 (2007).
Sutton, M.A. & Schuman, E.M. Dendritic protein synthesis, synaptic plasticity, and memory. Cell 127, 49–58 (2006).
Thiagarajan, T.C., Lindskog, M. & Tsien, R.W. Adaptation to synaptic inactivity in hippocampal neurons. Neuron 47, 725–737 (2005).
Cingolani, L.A. et al. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron 58, 749–762 (2008).
Gainey, M.A., Hurvitz-Wolff, J.R., Lambo, M.E. & Turrigiano, G.G. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J. Neurosci. 29, 6479–6489 (2009).
Wierenga, C.J., Ibata, K. & Turrigiano, G.G. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J. Neurosci. 25, 2895–2905 (2005).
Nuriya, M. & Huganir, R.L. Regulation of AMPA receptor trafficking by N-cadherin. J. Neurochem. 97, 652–661 (2006).
Cathala, L., Holderith, N.B., Nusser, Z., DiGregorio, D.A. & Cull-Candy, S.G. Changes in synaptic structure underlie the developmental speeding of AMPA receptor-mediated EPSCs. Nat. Neurosci. 8, 1310–1318 (2005).
Ryan, T.A. et al. The kinetics of SV recycling measured at single presynaptic boutons. Neuron 11, 713–724 (1993).
Arikkath, J. & Reichardt, L.F. Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci. 31, 487–494 (2008).
Gottmann, K. Transsynaptic modulation of the synaptic vesicle cycle by cell-adhesion molecules. J. Neurosci. Res. 86, 223–232 (2008).
Tanaka, H. et al. Molecular modification of N-cadherin in response to synaptic activity. Neuron 25, 93–107 (2000).
Acknowledgements
We thank R. Kemler (Max Planck Institute of Immunology) for β-catenin floxed mice, T. Okuda (Keio University) and M. Passafaro (University of Milan) for sharing plasmids, D. Elliott for expert technical assistance and the members of the Goda laboratory for discussions. This work was supported by the UK Medical Research Council, the European Union 7th Framework Program EUROSPIN project and the RIKEN Brain Science Institute.
Author information
Authors and Affiliations
Contributions
N.V. performed all of the experimental work. M.L. contributed electrophysiology experiments and discussion, and I.J.W. performed electron microscopy. N.V. and Y.G. designed the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 1251 kb)
Rights and permissions
About this article
Cite this article
Vitureira, N., Letellier, M., White, I. et al. Differential control of presynaptic efficacy by postsynaptic N-cadherin and β-catenin. Nat Neurosci 15, 81–89 (2012). https://doi.org/10.1038/nn.2995
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2995
- Springer Nature America, Inc.
This article is cited by
-
Microglial CD300f immune receptor contributes to the maintenance of neuron viability in vitro and after a penetrating brain injury
Scientific Reports (2023)
-
A delay in vesicle endocytosis by a C-terminal fragment of N-cadherin enhances Aβ synaptotoxicity
Cell Death Discovery (2023)
-
Synapse development and maturation at the drosophila neuromuscular junction
Neural Development (2020)
-
Glial ATP and Large Pore Channels Modulate Synaptic Strength in Response to Chronic Inactivity
Molecular Neurobiology (2020)
-
The cell adhesion protein CAR is a negative regulator of synaptic transmission
Scientific Reports (2019)