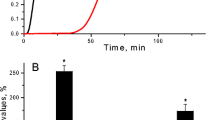
Abstract
The extensive homology between apolipoprotein(a) and plasminogen has led to the hypothesis that the increased risk for atherosclerosis, cardiac disease and stroke associated with elevated levels of apolipoprotein(a) may reflect modulation of fibrinolysis. We have investigated the role of apolipoprotein(a) on clot lysis in transgenic mice expressing the human apolipoprotein(a) gene. These mice develop fatty streak lesions resembling early lesions of human atherosclerosis. Pulmonary emboli were generated in mice by injection, through the right jugular vein, of a human platelet-rich plasma clot radiolabelled with technetium-99m-labelled antifibrin antibodies. Tissue plasminogen activator was introduced continuously via the right jugular vein. Clot lysis, determined by ex vivo imaging, was depressed in mice carrying the apolipoprotein(a) transgene relative to their sex-matched normal littermates. These results directly demonstrate an in vivo effect of apolipoprotein(a) on fibrinolysis, an effect that may contribute to the pathology associated with elevated levels of this protein.
Similar content being viewed by others
Change history
01 June 1995
HIV-speciflc cytotoxic T-cells in HIV-exposed but uninfected Gambian women S. Rowland-Jones, J. Sutton, K. Ariyoshi, T. Dong, F. Gotch, S. Mcadam, D. Whitby, S. Sabally, A. Gallimore, T. Corrah, M. Takiguchi, T. Schultz, Andrew Mcmichael & H. Whittle Nature Medicine 1, 59–64, 1995. An error in typography resulted in the incorrect printing of m for the Greek character μ.
References
Utermann, G. The mysteries of lipoproteln (a). Science 246, 904–910 (1989).
Scanu, A.M. & Fless, G.M. Lipoprotein (a): heterogeneity and biological significance. J. clin. Invest. 85, 1709–1715 (1990).
Scanu, A.M., Lawn, R.M. & Berg, K. Lipoprotein (a) and atherosclerosis: Davis Conference. Ann. intern. Med. 115, 209–218 (1991).
McLean, J.W. et. al. Human apolipoprotein (a): cDNA sequence of apolipoprotein (a) is homologous to plasminogen. Nature 300, 132–139 (1987).
Zysow, B.R. & Lawn, R.M. The relationship of lipoprotein (a) to hemostasis. Curr. Opin. Lipidology 4, 484–489 (1993).
Lawn, R.M. et. al. Atherogenesis in transgenic mice expressing human apolipoprotein (a). Nature 360, 670–672 (1992).
Chiesa, G. et. al. Reconstitution of lipoprotein (a) by infusion of human LDL into transgenic mice expressing human apolipoprotein (a). J. biol. Chem. 267, 24369–24374 (1992).
Hajjar, K.A., Gavish, D., Breslow, J.L. & Nachman, R.L. Lipoprotein (a) modulation of endothelial cell surface fibrinolysis and its potential role in atherosclerosis. Nature 339, 303–305 (1989).
Stassen, J.M., Lijnin, H.R., Kieckens, L. & Collen, D. Small animal thrombosis models for the evaluation of thrombolytic agents. Circulation, Suppl IV 83, 65–72 (1991).
Carmelliet, P. et. al. Plasminogen activator inhibitor-1 gene deficient mice. II. Effects on hemostasis, thrombosis and thrombolysis. J. clin. Invest. 92, 2756–2760 (1993).
Miles, L.A., Fless, G.M., Levin, E.G., Scanu, A.M. & Plow, E.F. A potential basis for the thrombotic risks associated with lipoprotein (a). Nature 339, 301–303 (1989).
Edelberg, J.M., Gonzalez-Gronow, M. & Pizzo, S.V. Lipoprotein (a) inhibition of plasminogen activation by tissue-type plasminogen activator. Thromb Res 57, 155–162 (1990).
Loscalzo, J., Weinfeld, M., Fless, G.M. & Scanu, A.M. Lipoprotein (a), fibrin binding, and plasminogen activation. Arteriosclerosis 10, 240–245 (1990).
Liu, J.N., Harpel, P.C., Pannell, R. & Gurewich, V. Lipoprotein (a): A kinetic study of its influence on fibrin-dependent plasminogen activation by prourokinase or tissue plasminogen activator. Biochemistry 32, 9694–9700 (1993).
Leerink, C. et. al. Lysine-binding heterogeneity of Lp (a): Consequences for fibrin binding and inhibition of plasminogen. Thromb. Haemost. 68. 185–188 (1992).
Halvorsen, S., Skjonsberg, O.H., Berg, K., Ruyter, R. & Godal, H.C. Lp (a) lipoprotein inhibit the fibrinolytic system? Thromb. Res. 68, 223–232 (1992).
Garcia-Frade, L.J. et. al. Fibrinolytic parameters and lipoprotein (a) levels in plasma of patients with coronary artery disease. Thromb. Res. 63, 407–418 (1991).
Heinrich, H., Sandkamp, M., Kokott, R., Schulte, H. & Assmann, G. Relationship of lipoprotein (a) to variables of coagulation and fibrinolysis in a healthy population. Clin. Chem. 37, 1950–1954 (1991).
Oshima, S. et al. Transient increase of plasma lipoprotein (a) in patients with unstable angina pectoris. Does lipoprotein (a) alter fibrinolysis. Arterioscler. Thromb. 11, 1772–1777 (1991).
Glueck, C.J. et. al. Relationship between lipoprotein (a), lipids, apolipoproteins, basal and stimulated fibrinolytic regulators, and D-dimer. Metabolism 42, 236–246 (1993).
Scanu, A.M., Praffinger, D., Lee, J.C. & Hinman, J. A single point mutation (Trp72→Arg) in human apo (a) kringle 4–37 associated with a lysine binding defect in Lp (a). Biochim. Biophys. Acta. 1227, 41–45 (1994).
von Hodenberg, E. et. al. Effects of lipoprotein (a) on success rate of thrombolytic therapy in acute myocardial infarction. Am. J. Cardiol. 67, 1349–1353 (1991).
Moliterno, D.J. et. al. Relation of plasma lipoprotein (a) to infarct artery patency in survivors of myocardial infarction. Circulation 88, 935–940 (1993).
Kudryk, B., Rohoza, A., Ahadi, M., Nechtin, J. & Wiebe, M.E. The specificity of a monoclonal antibody for the NH2-terminal region of fibrin. Molec. Immun. 21, 89–94 (1984).
Rosenbrough, S.F. et al Thrombus imaging with indium-111 and iodine-131-labelled fibrin-specific monoclonal antibody and its F (ab)'2 and Fab frag. J. nucl. Med. 29, 1212–1222 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Palabrica, T., Liu, A., Aronovitz, M. et al. Antifibrinolytic activity of apolipoprotein(a) in vivo: Human apolipoprotein(a) transgenic mice are resistant to tissue plasminogen activator-mediated thrombolysis. Nat Med 1, 256–259 (1995). https://doi.org/10.1038/nm0395-256
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm0395-256
- Springer Nature America, Inc.
This article is cited by
-
Lipoprotein(a), platelet function and cardiovascular disease
Nature Reviews Cardiology (2023)
-
Lipoprotein (a): Does It Play a Role in Pediatric Ischemic Stroke and Thrombosis?
Current Atherosclerosis Reports (2023)
-
Cholesterol Lowering Biotechnological Strategies: From Monoclonal Antibodies to Antisense Therapies. A Pre-Clinical Perspective Review
Cardiovascular Drugs and Therapy (2023)
-
Lipoprotein (a): Recent Updates on a Unique Lipoprotein
Current Atherosclerosis Reports (2021)
-
Relation of High Lipoprotein (a) Concentrations to Platelet Reactivity in Individuals with and Without Coronary Artery Disease
Advances in Therapy (2020)