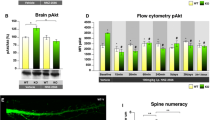
Abstract
Fragile X syndrome (FXS) is the leading monogenic cause of autism spectrum disorders (ASD). Trinucleotide repeat expansions in FMR1 abolish FMRP expression, leading to hyperactivation of ERK and mTOR signaling upstream of mRNA translation. Here we show that metformin, the most widely used drug for type 2 diabetes, rescues core phenotypes in Fmr1−/y mice and selectively normalizes ERK signaling, eIF4E phosphorylation and the expression of MMP-9. Thus, metformin is a potential FXS therapeutic.


Similar content being viewed by others
References
Gkogkas, C.G. et al. Cell Rep. 9, 1742–1755 (2014).
Hou, L. et al. Neuron 51, 441–454 (2006).
Dziembowska, M. et al. Am. J. Med. Genet. A. 161A, 1897–1903 (2013).
Leigh, M.J. et al. J. Dev. Behav. Pediatr. 34, 147–155 (2013).
Sidhu, H., Dansie, L.E., Hickmott, P.W., Ethell, D.W. & Ethell, I.M. J. Neurosci. 34, 9867–9879 (2014).
Foretz, M., Guigas, B., Bertrand, L., Pollak, M. & Viollet, B. Cell Metab. 20, 953–966 (2014).
Ming, M. et al. PLoS One 9, e114573 (2014).
Wang, J. et al. Cell Stem Cell 11, 23–35 (2012).
Soares, H.P., Ni, Y., Kisfalvi, K., Sinnett-Smith, J. & Rozengurt, E. PLoS One 8, e57289 (2013).
Łabuzek, K. et al. Pharmacol. Rep. 62, 956–965 (2010).
Hagerman, R., Au, J. & Hagerman, P. J. Neurodev. Disord. 3, 211–224 (2011).
Bhattacharya, A. et al. Neuron 76, 325–337 (2012).
Osterweil, E.K. et al. Neuron 77, 243–250 (2013).
Udagawa, T. et al. Nat. Med. 19, 1473–1477 (2013).
Li, J., Benashski, S.E., Venna, V.R. & McCullough, L.D. Stroke 41, 2645–2652 (2010).
Khang, R., Park, C. & Shin, J.H. Neurosci. Lett. 579, 145–150 (2014).
Jin, J. et al. Neuromolecular Med. 18, 581–592 (2016).
Singh, J., Olle, B., Suhail, H., Felicella, M.M. & Giri, S. J. Neurochem. 138, 86–100 (2016).
Lavoie, H. & Therrien, M. Nat. Rev. Mol. Cell Biol. 16, 281–298 (2015).
Berry-Kravis, E. et al. Sci. Transl. Med. 8, 321ra5 (2016).
Monyak, R.E. et al. Mol. Psychiatry (2016).
Forslund, K. et al. Nature 528, 262–266 (2015).
Hsiao, E.Y. et al. Cell 155, 1451–1463 (2013).
Gkogkas, C.G. et al. Nature 493, 371–377 (2013).
McKinney, B.C., Grossman, A.W., Elisseou, N.M. & Greenough, W.T. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 136B, 98–102 (2005).
Schmidt, E.K., Clavarino, G., Ceppi, M. & Pierre, P. Nat. Methods 6, 275–277 (2009).
Mogil, J.S. et al. Pain 126, 24–34 (2006).
Acknowledgements
This work is supported by the FRAXA Research Foundation, Brain Canada/FNC, a CIHR foundation grant (FDN-148423) and Brain & Behavior Research Foundation grants (24365) to N.S.; a Wellcome Trust/Royal Society Sir Henry Dale grant (107687/Z/15/Z) to C.G.G.; the Canada Research Chair Program (950-231066) to J.-C.L.; and a Brain Canada/NeuroDevNet Postdoctoral Training Award to J.P.
Author information
Authors and Affiliations
Contributions
I.G., A.K. and J.P. designed the experiments, performed data analysis and wrote the manuscript. I.G., A.K., J.P., A.A.-V., E.F., R.C., V.S., T.P., A.N., S.W., S.M.J., C.C., E.A.M. and C.G.G. designed and carried out experiments. A.S., V.T.T., I.A.G. and K.G. assisted with experiments. K.N. supervised the project. J.-C.L., C.G.G. and N.S. supervised the project, designed experiments and edited the manuscript. All authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures and Tables
Supplementary Figures 1–17 and Supplementary Tables 1 and 2. (PDF 5149 kb)
Rights and permissions
About this article
Cite this article
Gantois, I., Khoutorsky, A., Popic, J. et al. Metformin ameliorates core deficits in a mouse model of fragile X syndrome. Nat Med 23, 674–677 (2017). https://doi.org/10.1038/nm.4335
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4335
- Springer Nature America, Inc.
This article is cited by
-
SRC family kinase inhibition rescues molecular and behavioral phenotypes, but not protein interaction network dynamics, in a mouse model of Fragile X syndrome
Molecular Psychiatry (2024)
-
Beneficial effects of metformin treatment on memory impairment
Molecular Biology Reports (2024)
-
FMRP binds Per1 mRNA and downregulates its protein expression in mice
Molecular Brain (2023)
-
Phenotypic variability to medication management: an update on fragile X syndrome
Human Genomics (2023)
-
Akkermansia muciniphila, which is enriched in the gut microbiota by metformin, improves cognitive function in aged mice by reducing the proinflammatory cytokine interleukin-6
Microbiome (2023)