Abstract
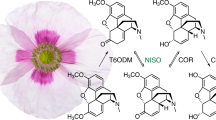
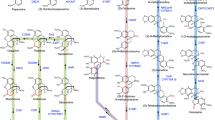
Two previously undetected enzymes involved in morphine biosynthesis and unique among plants to opium poppy have been identified as non-heme dioxygenases, in contrast to the functionally analogous cytochrome P450s found in mammals. We used functional genomics to isolate thebaine 6-O-demethylase (T6ODM) and codeine O-demethylase (CODM), the only known 2-oxoglutarate/Fe(II)-dependent dioxygenases that catalyze O-demethylation. Virus-induced gene silencing of T6ODM and CODM in opium poppy efficiently blocked metabolism at thebaine and codeine, respectively.


Similar content being viewed by others
References
Ziegler, J. & Facchini, P.J. Annu. Rev. Plant Biol. 59, 735–769 (2008).
Zhu, W. Med. Sci. Monit. 14, SC15–SC18 (2008).
Grobe, N. et al. J. Biol. Chem. 284, 24425–24431 (2009).
Unterlinner, B., Lenz, R. & Kutchan, T.M. Plant J. 18, 465–475 (1999).
Grothe, T., Lenz, R. & Kutchan, T.M. J. Biol. Chem. 276, 30717–30723 (2001).
Millgate, A.G. et al. Nature 431, 413–414 (2004).
Nyman, U. Hereditas 89, 43–50 (1978).
Hagel, J.M., Weljie, A.M., Vogel, H.J. & Facchini, P.J. Plant Physiol. 147, 1805–1821 (2008).
Cotterill, P. Method of altering the alkaloid composition in poppy plants. International patent WO/2005/107436 (2005).
Wilmouth, R.C. et al. Structure 10, 93–103 (2002).
Lister, D.L., Kanungo, G., Rathbone, D.A. & Bruce, N.C. FEMS Microbiol. Lett. 181, 137–144 (1999).
Craig, D.H., Moody, P.C.E., Bruce, N.C. & Scrutton, N.S. Biochemistry 37, 7598–7607 (1998).
Ishida, T., Yano, M. & Toki, S. Drug Metab. Dispos. 19, 895–899 (1991).
Tiainen, P., Myllyharju, J. & Koivunen, P. J. Biol. Chem. 280, 1142–1148 (2005).
Nielsen, B., Röe, J. & Brochmann-Hanssen, E. Planta Med. 48, 205–206 (1983).
Brochmann-Hanssen, E. Planta Med. 50, 343–345 (1984).
De Carolis, E. & De Luca, V. Phytochemistry 36, 1093–1107 (1994).
Hausinger, R.P. Crit. Rev. Biochem. Mol. Biol. 39, 21–68 (2004).
Clifton, I.J. et al. J. Inorg. Biochem. 100, 644–669 (2006).
Loenarz, C. & Schofield, C.J. Nat. Chem. Biol. 4, 152–156 (2008).
Rapoport, R., Hanukoglu, I. & Sklan, D. Anal. Biochem. 218, 309–313 (1994).
International Narcotics Control Board. Narcotic drugs: estimated world requirements for 2007; statistics for 2005 (E/INCB/2006/2). (United Nations Publications, 2006).
Hawkins, K.M. & Smolke, C.D. Nat. Chem. Biol. 4, 564–573 (2008).
Minami, H. et al. Proc. Natl. Acad. Sci. USA 105, 7393–7398 (2008).
Parker, H.I., Blaschke, G. & Rapoport, H. J. Am. Chem. Soc. 94, 1276–1282 (1972).
Acknowledgements
We are grateful to D. Kumar (Yale University) for the pTRV1 and pTRV2 vectors, V. Irish (Yale University) for the pTRV2-PapPDS construct, the Canadian National Research Council Plant Biotechnology Institute for hosting our sequence data on their FIESTA2 annotation platform, and Sanofi-Aventis for the gift of the opium poppy varieties and the alkaloid standards used in this work. We also thank K. Zulak, R. Bourgault and J. Ziegler for technical assistance with cDNA library construction, microarray preparation and mass spectrometry, respectively. J.M.H. is the recipient of an Alberta Ingenuity Graduate Scholarship. Funding for this work was provided through a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada and a Canada Research Chair in Plant Metabolic Processes Biotechnology, both awarded to P.J.F.
Author information
Authors and Affiliations
Contributions
J.M.H. and P.J.F. contributed equally to all aspects of the experimental design and execution, and the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13, Supplementary Tables 1–3 and Supplementary Methods (PDF 1475 kb)
Rights and permissions
About this article
Cite this article
Hagel, J., Facchini, P. Dioxygenases catalyze the O-demethylation steps of morphine biosynthesis in opium poppy. Nat Chem Biol 6, 273–275 (2010). https://doi.org/10.1038/nchembio.317
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.317
- Springer Nature America, Inc.
This article is cited by
-
The effects of green and chemically-synthesized copper oxide nanoparticles on the production and gene expression of morphinan alkaloids in Oriental poppy
Scientific Reports (2024)
-
A flavonol synthase (FLS) gene, GhFLS1, was screened out increasing salt resistance in cotton
Environmental Sciences Europe (2023)
-
A Catharanthus roseus Fe(II)/α-ketoglutarate-dependent dioxygenase catalyzes a redox-neutral reaction responsible for vindolinine biosynthesis
Nature Communications (2022)
-
Insights into opium poppy (Papaver spp.) genetic diversity from genotyping-by-sequencing analysis
Scientific Reports (2022)
-
Engineering Saccharomyces cerevisiae to produce plant benzylisoquinoline alkaloids
aBIOTECH (2021)