Abstract
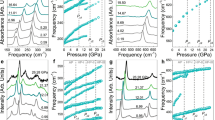
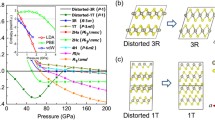
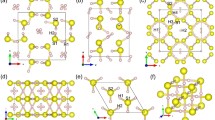
The application of pressure, internal or external, transforms molecular solids into extended solids with more itinerant electrons to soften repulsive interatomic interactions in a tight space. Examples include insulator-to-metal transitions in O2, Xe and I2, as well as molecular-to-non-molecular transitions in CO2 and N2. Here, we present new discoveries of novel two- and three-dimensional extended non-molecular phases of solid XeF2 and their metallization. At ∼50 GPa, the transparent linear insulating XeF2 transforms into a reddish two-dimensional graphite-like hexagonal layered structure of semiconducting XeF4. Above 70 GPa, it further transforms into a black three-dimensional fluorite-like structure of the first observed metallic XeF8 polyhedron. These simultaneously occurring molecular-to-non-molecular and insulator-to-metal transitions of XeF2 arise from the pressure-induced delocalization of non-bonded lone-pair electrons to sp3d2 hybridization in two-dimensional XeF4 and to p3d5 in three-dimensional XeF8 through the chemical bonding of all eight valence electrons in Xe and, thereby, fulfilling the octet rule at high pressures.





Similar content being viewed by others
References
Bartlett, N. Xenon hexafluoroplatinate(V) Xe+[PtF6]−. Proc. Chem. Soc. 218 (1962).
Graham, L., Graudejus, O., Jha, N. K. & Bartlett, N. Concerning the nature of XePtF6 . Coord. Chem. Rev. 197, 321–334 (2000).
Grochala, W. Atypical compounds of gases, which have been called ‘noble’. Chem. Soc. Rev. 36, 1632–1655 (2007).
Tramsek, M. & Zemva, B. Synthesis, properties and chemistry of xenon(II) fluoride. Acta Chim. Slov. 53, 105–116 (2006).
Brown, E. C., Cohen, A. & Gerber, R. Prediction of a linear polymer made of xenon and carbon. J. Chem. Phys. 122, 171101 (2005).
Pauling, L. Angles between orthogonal spd bond orbitals with maximum strength. Proc. Natl Acad. Sci. 73, 1403–1405 (1976).
Dixon, D. A., de Jong, W. A., Peterson, K. A., Christe, K. O. & Schrobilgen, G. J. Heats of formation of xenon fluorides and the fluxionality of XeF6 from high level electronic structure calculations. J. Am. Chem. Soc. 127, 8627–8634 (2005).
Jortner, J., Rice, S. A. & Wilson, E. G. Speculation concerning the nature of binding in xenon fluorine compounds. J. Chem. Phys. 38, 2302–2303 (1963).
Agron, P. A. et al. F. Xenon difluoride and the nature of the xenon–fluorine bond. Science 139, 842–844 (1963).
Grochala, W., Hoffmann, R., Feng, J. & Ashcroft, N. W. The chemical imagination at work in very tight places. Angew. Chem. Int. Ed. 46, 3620–3642 (2007).
Sidgwick, N. V. & Powell, H. M. Bakerian lecture. Stereochemical types and valency groups. Proc. R. Soc. Lond. A 176, 153–180 (1940).
Gillespie, R. J. & Nyholm, R. S. Inorganic stereochemistry. Q. Rev. Chem. Soc. 11, 339–380 (1957).
Andersson, S. On the stereochemistry of valence bonds and the structures of XeO3, XeF4 and XeF2 . Acta Cryst. B35, 1321–1324 (1979).
Schwarz, U. & Syassen, K. The behavior of solid XeF2 under pressure. High Pressure Res. 9, 47–50 (1992).
Ashcroft, N. W. Hydrogen dominant metallic alloys: high temperature superconductors? Phys. Rev. Lett. 92, 187002 (2004).
Eremets, M. I., Triojan, I. A., Medvedev, S. A., Tse, J. S. & Yao, Y. Superconductivity in hydrogen dominant materials: silane. Science 319, 1506–1509 (2008).
Reichlin, R. et al. Evidence for the insulator–metal transition in xenon from optical, X-ray and band-structure studies to 170 GPa. Phys. Rev. Lett. 62, 669–672 (1989).
Goettel, K. A., Eggert, J. H., Silvera, I. F. & Moss, W. C. Optical evidence for the matallization of xenon at 132(5) GPa. Phys. Rev. Lett. 62, 665–668 (1989).
Takemura, K., Sato, K., Fujihisa, H. & Onoda, M. Modulated structure of solid iodine during its molecular dissociation under high pressure. Nature 423, 971–974 (2003).
Fujihisa, H., Fujii, Y., Takemura, K. & Shimomura, O. Structural aspects of dense solid halogens under high pressure studied by X-ray diffraction—molecular dissociation and metallization. J. Phys. Chem. Solids 56, 1439–1444 (1995).
Burbank, R. D., Falconer, W. E. & Sunder, W. A. Crystal structure of krypton difluoride at −80 °C. Science 178, 1285–1286 (1972).
Robinson, E. A., Johnson, S. A., Tang, T.-H. & Gillespie, R. J. Reinterpretation of the lengths of bonds to fluorine in terms of an almost ionic model. Inorg. Chem. 36, 3022–3030 (1997).
Reich, S. & Thomsen, C. Raman spectroscopy of graphite. Phil. Trans. R. Soc. Lond. A 362, 2271–2288 (2004).
Eremets, M. I. et al. Superconductivity of Xe at Mbar pressure. Phys. Rev. Lett. 85, 2797–2800 (2000).
Eremets, M. I., Struzhkin, V. V., Mao, H. K. & Hemley, R. J. Superconductivity in boron. Science 293, 272–274 (2001).
Shimizu, K., Ishikawa, H., Takao, D., Yagi, T. & Amaya, K. Superconductivity in compresed Li at 20 K. Nature 419, 597–599 (2002).
Qazilbash, M. M. et al. Mott transition in VO2 revealed by infrared spectroscopy and nano-image. Science 318, 1750–1553 (2007).
Acknowledgements
Synchrotron X-ray diffraction studies were carried out at the GSECAR 13IDD and HPCAT 16BMD beam line at the Advanced Photon Source. The authors thank P. Dera, V. Prakapenka, O. Shebanova and A. Sengupta for their technical support. The present study was supported by DTRA (grant no. HDTRA1-09-1-0041), NSF-DMR (grant no. 0854618) and DOE-NNSA (no. DE-F603-97SF21388).
Author information
Authors and Affiliations
Contributions
M.K. carried out X-ray and Raman measurements and performed the analysis, including calculating the electronic structure. M.D. performed resistance measurements and analysis. C.-S.Y. was responsible for the overall design, direction and supervision of the project. All authors contributed to writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2283 kb)
Rights and permissions
About this article
Cite this article
Kim, M., Debessai, M. & Yoo, CS. Two- and three-dimensional extended solids and metallization of compressed XeF2. Nature Chem 2, 784–788 (2010). https://doi.org/10.1038/nchem.724
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.724
- Springer Nature Limited
This article is cited by
-
Ag9GaSe6: high-pressure-induced Ag migration causes thermoelectric performance irreproducibility and elimination of such instability
Nature Communications (2022)
-
Open questions on the high-pressure chemistry of the noble gases
Communications Chemistry (2022)
-
Reactivity of He with ionic compounds under high pressure
Nature Communications (2018)
-
On the position of helium and neon in the Periodic Table of Elements
Foundations of Chemistry (2018)
-
Phase Diagram and Transformations of Iron Pentacarbonyl to nm Layered Hematite and Carbon-Oxygen Polymer under Pressure
Scientific Reports (2015)