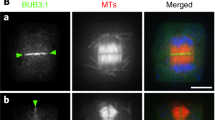
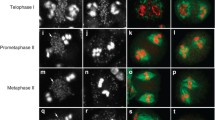
Abstract
MOR1 is a member of the MAP215 family of microtubule-associated proteins and is required to establish interphase arrays of cortical microtubules in plant cells1. Here we show that MOR1 binds microtubules in vivo, localizing to both cortical microtubules and to areas of overlapping microtubules in the phragmoplast. Genetic complementation of the cytokinesis-defective gemini pollen 1-1 (gem1-1) mutation with MOR1 shows that MOR1 (which is synonymous with the protein GEM1) is essential in cytokinesis. Phenotypic analysis of gem1-1 and gem1-2, which contains a T-DNA insertion, confirm that MOR1/GEM1 is essential for regular patterns of cytokinesis. Both the gem1-1 and gem1-2 mutations cause the truncation of the MOR1/GEM1 protein. In addition, the carboxy-terminal domain of the protein, which is absent in both mutants, binds microtubules in vitro. Our data show that MOR1/GEM1 has an essential role in the cytokinetic phragmoplast.




Similar content being viewed by others
References
Whittington, A. T. et. al. Nature 411, 610–613 (2000).
Smertenko, A. et. al. Nature Cell Biol. 2, 750–753 (2000).
Igarashi, H. et. al. Plant Cell Physiol. 41, 920–931 (2000).
Hussey, P. J., Hawkins, T. J., Igarashi, H., Kaloriti, D. & Smertenko, A. Plant Mol. Biol. (in the press).
Hussey, P. J. & Hawkins, T. J. Trends Plant Sci. 6, 389–392 (2001).
Charrasse, S. et. al. J. Cell Sci. 111, 1371–1383 (1998).
Graef, R., Daunderer, C. & Schliwa, M. J. Cell Sci. 113, 1747–1758 (2000).
Cullen, C. F., Deah, P., Glover, D. M. & Ohkura, H. et. al. J. Cell Biol. 146, 1005–1018 (1999).
Hirsh, D. & Vanderslice, R. Dev. Biol. 49, 220–235 (1979).
Ohkura, H. et. al. EMBO J. 7, 1465–1473 (1988).
Garcia M. A., Vardy, L., Koonrugsa, N. & Toda, T. EMBO J. 20, 3389–3401 (2001).
Wang P. J., and Huffaker, T. C. J. Cell. Biol. 139, 1271–1280 (1997).
Tournebize, R. et. al. Nature Cell Biol. 2, 13–19 (2000).
Neuwald, A. F. & Hirano, T. Genome Res. 10, 1445–1452 (2000).
Twell, D., Park, S. K. & Lalanne, E. Trends Plant Sci. 3, 305–310 (1998).
Park, S. K., Howden, R. & Twell, D. Development 125, 3789–3799 (1998).
Park, S. K. & Twell, D. Plant Physiol. 126, 899–909 (2001).
Popov, A. V. et. al. EMBO J. 20, 397–410 (2001).
Asada, T., Sonobe, S. & Shibaoka, H. Nature 350, 238–241 (1991).
Gard D. L. & Kirschner, M. W. J. Cell Biol. 105, 2203–2215 (1987).
Vasquez, R. J., Gard, D. L. & Cassimeris, L. J. Cell Biol. 127, 985–993 (1994).
Tournebize, R. et. al. Nature Cell Biol. 2, 13–19 (2000).
Smertenko, A. P., Lawrence, S. L. & Hussey, P. J. Protoplasma. 203, 138–143 (1998).
Shelanski M. L., Gaskin, F. & Cantor, C. R. Proc. Natl Acad. Sci. USA 70, 765–768 (1973).
Clough, S. J. & Bent, A. F. Plant J. 16, 735–743 (1998).
Goddijn, O. J. M. et. al. Plant J. 4, 863–873 (1993).
Acknowledgements
We thank G. Wasteneys for the MOR1 sequencing primers. This work was funded by the Biotechnology and Biological Sciences Research Council UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary figures
Figure S1 (PDF 678 kb)
Figure S2
Figure S3
Figure S4
Rights and permissions
About this article
Cite this article
Twell, D., Park, S., Hawkins, T. et al. MOR1/GEM1 has an essential role in the plant-specific cytokinetic phragmoplast. Nat Cell Biol 4, 711–714 (2002). https://doi.org/10.1038/ncb844
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb844
- Springer Nature Limited
This article is cited by
-
Mutation in Arabidopsis MOR1 gene impairs endocytosis in stamen filament cells and results in anther indehiscence
Plant Growth Regulation (2022)
-
OsAPT1 a Pollen Preferentially Expressed Gene Is Essential for Pollen Tube Germination and Elongation in Rice
Plant Molecular Biology Reporter (2021)
-
Identification of New Mutant Alleles of Augmin Subunits Broadens Spectrum of Augmin Function During Sexual Reproduction in Arabidopsis
Journal of Plant Biology (2020)
-
Building new insights in plant gametogenesis from an evolutionary perspective
Nature Plants (2019)
-
Male gametophyte development and function in angiosperms: a general concept
Plant Reproduction (2016)