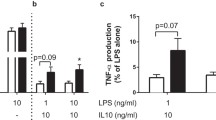
Abstract
Objective:
Interleukin-18 (IL-18) has been recently demonstrated to improve experimental hyperphagia and insulin resistance. Paradoxically, concentrations of circulating IL-18 in obese subjects and in patients with type 2 diabetes are increased. The objective of this study is to provide an explanation for this paradox.
Design:
We have hypothesized that cells from obese individuals or from patients with type 2 diabetes mellitus have a diminished response to stimulation with IL-18. IL-18 responsiveness was tested by stimulating blood monocytes of obese or diabetes patients with rIL-18 or microbial components.
Results:
Obese individuals and patients with type 2 diabetes mellitus exhibit increased circulating concentrations of IL-18. More importantly, leukocytes isolated from obese or type 2 diabetes patients respond poorly after stimulation with IL-18, as reflected by defective interferon-γ (IFNγ) production. The defective response to IL-18 stimulation was accompanied by a 50% reduction in the expression of IL-18R α and β chains. In addition, cells of patients with obesity and diabetes displayed an impaired release of IFNγ after challenge with bacterial or fungal pathogens, which was due to defective IL-18-mediated signaling.
Conclusion:
Patients with obesity or type 2 diabetes mellitus are characterized by lower responses after stimulation with IL-18. This IL-18 resistance explains the association of obesity and diabetes with high IL-18 circulating concentrations, similar to hyperinsulinemia and hyperleptinemia. IL-18 resistance may represent an important mechanism of the increased susceptibility of these patients to a number of infections.




Similar content being viewed by others
References
Bazan JF, Timans JC, Kastelein RA . A newly defined interleukin-1? Nature 1996; 379: 591.
Dinarello CA, Novick D, Puren AJ, Fantuzzi G, Shapiro L, Mühl H et al. Overview of interleukin-18: more than an interferon-gamma inducing factor. J Leuk Biol 1998; 63: 658–664.
Fantuzzi G, Dinarello CA . Interleukin-18 and interleukin-1beta: two cytokine susbstrates for ICE (caspase-1). J Clin Immunol 1999; 19: 1–11.
Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A et al. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature 1997; 386: 619–623.
Hung J, McQuillan BM, Chapman CM, Thompson PL, Beilby JP . Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol 2005; 25: 1268–1273.
Fischer CP, Perstrup LB, Berntsen A, Eskildsen P, Pedersen BK . Elevated plasma interleukin-18 is a marker of insulin-resistance in type 2 diabetic and non-diabetic humans. Clin Immunol 2005; 117: 152–160.
Escobar-Morreale HF, Botella-Carretero JI, Villuendas G, Sancho J, San Millan JL . Serum interleukin-18 concentrations are increased in the plycystic ovary syndrome: relationship to insulin resistance and obesity. J Clin Endocrinol Metab 2004; 89: 806–811.
Bruun JM, Stallknecht B, Helge JW, Richelsen B . Interleukin-18 in plasma and adipose tissue: effects of obesity, insulin resistance, and weight loss. Eur J Endocrinol 2007; 157: 465–471.
Leick L, Lindegaard B, Stensvold D, Plomgaard P, Saltin B, Pilegaard H . Adipose tissue interleukin-18 mRNA and plasma interleukin-18: effect of obesity and exercise. Obesity 2007; 15: 356–363.
Thompson SR, Sanders J, Stephens JW, Miller GJ, Humphries SE . A common interleukin 18 haplotype is associated with higher body mass index in subjects with diabetes and coronary heart disease. Metabolism 2007; 56: 662–669.
Vilarrasa N, Vendrell J, Sanchez-Santos R, Broch M, Megia A, Masdevall C et al. Effect of weight loss induced by gastric bypass on proinflammatory interleukin-18, soluble tumour necrosis factor-alpha receptors, C-reactive protein and adiponectin in morbidly obese patients. Clin Endocrinol 2007; 67: 679–686.
Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med 2006; 12: 650–656.
Zorrilla EP, Sanchez-Alavez M, Sugama S, Brennan M, Fernandez R, Bartfai T et al. Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci USA 2007; 104: 11097–11102.
Joshi N, Caputo GM, Weitekamp MR, Karchmer AW . Infections in patients with diabetes mellitus. N Engl J Med 1999; 341: 1906–1912.
Falagas ME, Kompoti M . Obesity and infection. Lancet Infect Dis 2006; 6: 438–446.
Hopkins PN, Hunt SC, Wu LL, Williams GH, Williams RR . Hypertension, dyslipidemia and insulin resistance: links in a chain or spokes on a wheel ? Curr Opin Lipidol 1996; 7: 241–253.
Root HF . The association of diabetes and tuberculosis. N Engl J Med 1934; 210: 1–13.
Alisjahbana B, van Crevel R, Sahiratmadja E, den Heijer M, Maya A, Istriana E et al. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int J Tuberc Lung Dis 2006; 10: 696–700.
Valerius NH, Eff C, Hansen NE, Karle H, Nerup J, Søeberg B et al. Neutrophil and lymphocyte function in patients with diabetes mellitus. Acta Med Scand 1982; 211: 463–467.
Delamaire M, Maugendre D, Moreno M, Le Goff M-C, Allanic H, Genetet B . Impaired leukocyte functions in diabetic patients. Diabetic Med 1997; 14: 29–34.
Gallacher SJ, Thomson G, Fraser WD, Fisher BM, Gemmell CG, MacCuish AC . Neutrophil bactericidal function in diabetes mellitus: evidence for association with blood glucose control. Diabet Med 1995; 12: 916–920.
Tsiavou A, Hatziagelaki E, Chaidaroglou A, Koniavitou K, Degiannis D, Raptis SA . Correlation between intracellular interferon-gamma (IFN-gamma) production by CD4+ and CD8+ lymphocytes and IFN-gamma gene polymorphism in patients with type 2 diabetes mellitus and latent autoimmune diabetes of adults (LADA). Cytokine 2005; 31: 135–141.
Murray HW . Interferon-gamma and host antimicrobial defense: current and future clinical applications. Am J Med 1994; 97: 459–467.
Fontana L, Eagon JC, Colonna M, Klein S . Impaired mononuclear cell immune function in extreme obesity is corrected by weight loss. Rejuvenation Res 2007; 10: 41–46.
Tsiavou A, Degiannis D, Hatziagelaki E, Koniavitou K, Raptis S . Flow cytometric detection of intracellular IL-12 release: in vitro effect of widely used immunosuppressants. Int Immunopharmacol 2002; 2: 1713–1720.
Tsukaguchi K, Okamura H, Ikuno M, Kobayashi A, Fukuoka A, Takenaka H et al. [The relation between diabetes mellitus and IFN-gamma, IL-12 and IL-10 productions by CD4+ alpha beta T cells and monocytes in patients with pulmonary tuberculosis]. Kekkaku 1997; 72: 617–622.
Grebenchtchikov N, van der Ven-Jongekrijg J, Pesman GJ, Geurts-Moespot A, van der Meer JW, Sweep FC . Development of a sensitive ELISA for the quantification of human tumour necrosis factor-alpha using 4 polyclonal antibodies. Eur Cytokine Netw 2005; 16: 215–222.
Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J Immunol 1998; 161: 3400–3407.
Draznin B . Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: the two sides of a coin. Diabetes 2006; 55: 2392–2397.
Karlsson HK, Zierath JR . Insulin signaling and glucose transport in insulin resistant human skeletal muscle. Cell Biochem Biophys 2007; 48: 103–113.
Inoue H, Ogawa W, Ozaki M, Haga S, Matsumoto M, Furukawa K et al. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nature Med 2004; 10: 168–174.
Netea MG, Joosten LAB, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ et al. Deficiency of interleukin-18 in mice leads to obesity, insulin resistance, and hyperglycemia, through defective STAT3-mediated signaling. Nature Med 2006; 12: 650–656.
Kalina U, Kauschat D, Koyama N, Nuernberger H, Ballas K, Koschmieder S et al. IL-18 activates STAT3 in the Natural Killer cell line 92, augments cytotoxic activity, and mediates IFN production by the stress kinase p38 and by the extracellular regulated kinases p44/erk-1 and p42/erk-21. J Immunol 2000; 165: 1307–1313.
Sugimoto N, Nakahira M, Ahn HJ, Micallef M, Hamaoka T, Kurimoto M et al. Differential requirements for JAK2 and TYK2 in T cell proliferation and IFN-gamma production induced by IL-12 alone or together with IL-18. Eur J Immunol 2003; 33: 243–251.
Kojima H, Takeuchi M, Ohta T, Nishida Y, Arai N, Ikeda M et al. Interleukin-18 activates the IRAK-TRAF6 pathway in mouse EL-4 cells. Biochem Biophys Res Commun 1998; 244: 183–186.
Kumar A, Takada Y, Boriek AM, Aggarwal BB . Nuclear factor-kappaB: its role in health and disease. J Mol Med 2004; 82: 434–448.
Koziel H, Koziel MJ . Pulmonary complications of diabetes mellitus: pneumonia. Infect Dis Clin North Am 1995; 9: 65–96.
Vellenga E, Uyl-de Groot CA, de Wit R, Keizer HJ, Löwenberg B, ten Haaft MA et al. Randomized placebo-controlled trial of granulocyte-macrophage colony-stimulating factor in patients with chemotherapy-related febrile neutropenia. J Clin Oncol 1996; 14: 619–627.
Sentochnik DE . Deep soft-tissue infections in diabetic patients. Infect Dis Clin North Am 1995; 9: 53–64.
Acknowledgements
MGN was supported by a Vidi-grant of the Netherlands Organization for Scientific Research. The research in this paper was partly funded by a grant from the Dutch Diabetes Research Foundation. CJT was partly funded by NIDDK, DK069881.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zilverschoon, G., Tack, C., Joosten, L. et al. Interleukin-18 resistance in patients with obesity and type 2 diabetes mellitus. Int J Obes 32, 1407–1414 (2008). https://doi.org/10.1038/ijo.2008.109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2008.109
- Springer Nature Limited
Keywords
This article is cited by
-
Role of IL-18 in adipose tissue remodeling and metabolic dysfunction
International Journal of Obesity (2024)
-
Circulating Cytokine Levels and Cardiovascular Disease Risk Profile in Young Adult Offspring of Women with Type 1 Diabetes
Diabetes Therapy (2023)
-
Expression of pro-inflammatory cytokines (IL-6 & IL-18) exacerbate the risk of diabetic nephropathy in the Pakistani population
Molecular Biology Reports (2023)
-
Interleukin-6 is a better metabolic biomarker than interleukin-18 in young healthy adults
Journal of Physiology and Biochemistry (2015)
-
Obesity development in caspase-1-deficient mice
International Journal of Obesity (2014)