Abstract
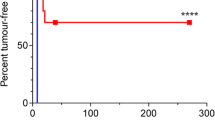
To test the anti-tumour activity of rhizoxin in recurrent and/or metastatic squamous cell head and neck cancer, we performed a phase II study. Eligibility required histologically proven squamous cell head and neck cancer. Patients could only have received one prior chemotherapy. Patients were entered if WHO PS was < or = 2 and organ functions were normal. Treatment consisted of rhizoxin 1.5-2.0 mg m-2 i.v. bolus injection once every 3 weeks. Thirty-two patients entered the study. All were eligible, 31 were evaluable for toxicity and 25 for response. Toxicity mainly consisted of pain at the tumour site and leucocytopenia. Mild asthenia and stomatitis were also observed. Two objective partial responses, lasting 7.5 and 3.5 months, were seen. Rhizoxin at this dose and schedule has minor activity in recurrent and/or metastatic squamous cell head and neck cancer.
Similar content being viewed by others
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Verweij, J., Wanders, J., Gil, T. et al. Phase II study of rhizoxin in squamous cell head and neck cancer. Br J Cancer 73, 400–402 (1996). https://doi.org/10.1038/bjc.1996.69
Issue Date:
DOI: https://doi.org/10.1038/bjc.1996.69
- Springer Nature Limited