Abstract
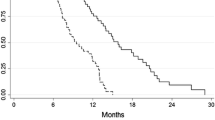
High response rates have been reported in the treatment of advanced gastric cancer with epirubicin, cisplatin and continuous infusion 5-fluorouracil (ECF), including instances of unresectable disease being rendered operable by chemotherapy. We report our experience with ECF as neoadjuvant treatment in gastric and lower oesophageal carcinoma. Twenty-seven patients were treated, of whom ten (37%) had carcinoma of the stomach and 17 (63%) tumours of the lower oesophagus. Histology in the majority of cases, 21 (78%), was adenocarcinoma. Before chemotherapy ten patients (37%) had evidence of initially unresectable locally advanced disease, 16 (59%) had localised disease only and one patient (4%) had a localised primary with a single liver metastasis. Epirubicin (50 mg m(-2) i.v.) and cisplatin (60 mg m(-2) i.v.) were administered every 3 weeks for four cycles together with a continuous 12 week infusion of 5-fluorouracil (200 mg m(-2) day(-1)). Fifteen of 24 assessable patients (62%) had symptomatic improvement on chemotherapy. On combined surgical and/or radiological assessment, 15 of the 27 patients (56%) had objective evidence of tumour response. In all patients assessment for radical surgery was made following chemotherapy. Eighteen patients (67%) proceeded to operation: of these, 11 had complete resection of their disease, one had a histologically incomplete resection and six were found to have unresectable disease. No pathological complete responses were observed. Only one of the ten patients with locally advanced disease achieved complete surgical resection after chemotherapy. At a median follow-up of 36 months from date of diagnosis (range 30-47 months), 19 of the 27 patients (70%) have died. Of 11 patients who had a complete surgical resection, one died post-operatively, three have subsequently relapsed (of whom two have died) and seven remain disease free. Toxicity from treatment was mild and included emesis, myelosuppression, stomatitis and exfoliation. Myelosuppression caused modification of treatment in 14 of 108 chemotherapy cycles (13%). There was one surgical death but no chemotherapy-related deaths. These early results show encouraging symptomatic and objective responses of gastro-oesophageal carcinoma to ECF, but provide no instances of ECF achieving complete pathological response. Only randomised trials can establish the role of neoadjuvant ECF chemotherapy in both initially resectable and unresectable carcinoma of the stomach and lower oesophagus.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Melcher, A., Mort, D. & Maughan, T. Epirubicin, cisplatin and continuous infusion 5-fluorouracil (ECF) as neoadjuvant chemotherapy in gastro-oesophageal cancer. Br J Cancer 74, 1651–1654 (1996). https://doi.org/10.1038/bjc.1996.604
Issue Date:
DOI: https://doi.org/10.1038/bjc.1996.604
- Springer Nature Limited
This article is cited by
-
Pathological evaluation of neoadjuvant chemotherapy in advanced gastric cancer
World Journal of Surgical Oncology (2019)
-
Comparison of efficacy of different route of administration of chemotherapy on unresectable, advanced gastric cancer
World Journal of Surgical Oncology (2012)
-
Retrospective analysis of 45 consecutive patients with advanced gastric cancer treated with neoadjuvant chemotherapy using an S-1/CDDP combination
Gastric Cancer (2006)
-
High curative resection rate with weekly cisplatin, 5-fluorouracil, epidoxorubicin, 6S-leucovorin, glutathione, and filgastrim in patients with locally advanced, unresectable gastric cancer: a report from the Italian Group for the Study of Digestive Tract Cancer (GISCAD)
British Journal of Cancer (2004)
-
Gastric carcinoma
Current Oncology Reports (2004)