Abstract
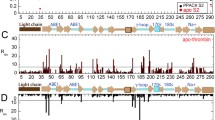
A glycosylated fragment of thrombomodulin containing two epidermal growth factor-like domains (TMEGF45) was analyzed by NMR. The 4th-domains structure of this two-domain fragment is similar to that of the individual domain previously determined. The 5th-domain, which has uncrossed disulfide bonds, is not as well determined in the two-domain fragment than the individual domain previously solved. The flexibility of the 5th-domain is consistent with low heteronuclear NOEs. In the individual 5th-domain, Met 388 was disordered, and key thrombin-binding residues formed a hydrophobic core. By contrast, in TMEGF45, Met 388 is in the 5th-domain core, positioned by Phe 376 from the 4th-domain. As a result, key thrombin-binding residues that were in the core of the individual domain are expelled. Upon thrombin binding, chemical shifts of two residues in the 4th-domain, the three interdomain linker residues, and nearly all of the 5th-domain are perturbed. Thus, TMEGF45 binds thrombin by an induced fit mechanism involving a flexible 5th-domain.




Similar content being viewed by others
Accession codes
References
Esmon, C.T. J. Biol. Chem. 264, 4743–4746 (1989).
Esmon, N.L. Prog. Hemost. Throm. 9, 29–55 (1989).
White, C.E., Hunter, M.J., Meininger, D.P., White, L.R. & Komives, E.A. Protein Eng. 8, 1177–1187 (1995).
Meininger, D.P., Hunter, M.J. & Komives, E.A. Protein Sci. 4, 1683–1695 (1995).
Sampoli Benitez, B.A., Hunter, M.J., Meininger, D.P. & Komives, E.A. J. Mol. Biol. 273, 913–926 (1997).
Nagashima, M., Lundh, E., Leonard, J.C., Morser, J. & Parkinson, J.F. J. Biol. Chem. 268, 2888–2892 (1993).
Clarke, J.H., et al. J. Biol. Chem. 268, 6309–6315 (1993).
Glaser, C.B. et al. J. Clin. Invest. 90, 2565–2573 (1992).
Hunter, M.J. & Komives, E.A., Protein Sci. 4, 2129–2137 (1995).
White, C.E., Hunter, M.J., Meininger, D.P., Garrod, S. & Komives, E.A., Proc. Natl. Acad. Sci. 93, 10177–10182 (1996).
Downing, A.K. et al. Cell 85, 597–605 (1996).
Morgan, W.D., et al. J. Mol. Biol. 289, 113–122 (1999).
Brandstettler, H., Bauer, M., Huber, R., Lollar, P. & Bode, W., Proc. Natl. Acad. Sci. 92, 9796–9800 (1995).
Pike, A.C.W., Brzozowski, A.M., Roberts, S.M., Olsen, O.H. & Persson, E., Proc. Natl. Acad. Sci. 96, 8925–8930 (1999).
Wishart, D.S., Bigam, C.G., Holm, A., Hodges, R.S. & Sykes, B.D., J.Biomol. NMR 5, 67–81 (1995).
Jacobsen, N.E., et al. Biochemistry 35, 3402–3417 (1996).
Hrabal, R., Komives, E.A. & Ni, F., Protein Sci. 5, 195–203 (1996).
Esmon, C.T., FASEB J. 9, 946–950 (1995).
Holmbeck, S.M.A., Dyson, H.J. & Wright, P.E., J. Mol. Biol. 284, 533–539 (1998).
Jin, C., Marsden, I., Chen, X. & Liao, X., J. Mol. Biol. 289, 683–690 (1999).
van Tilborg, P.J., et al. Biochemistry 38, 1951–1956 (1999).
Wikstrom, A., Berglund, H., Hambraeus, C., van den Berg, S. & Hard, T., J. Mol. Biol. 289, 963–979 (1999).
Baerga-Ortiz, A., Rezaie, A.R. & Komives, E.A., J. Mol. Biol. 296, 651–658 (2000).
Wood, M.J. & Komives, E.A., J. Biomol. NMR 13, 149–159 (1999).
Ni, F., Konishi, Y. & Scheraga, H.A., Biochemistry 29, 4479–4489 (1990).
Ye, J., Esmon, N.L., Esmon, C.T. & Johnson, A.E., J. Biol. Chem. 266, 23016–23021 (1991).
Vuister, G.W. & Bax, A., J. Am. Chem. Soc. 115, 7772–7777 (1993).
Nooren, I.M.A., et al. Biochemistry 38, 6035–6042 (1999).
Brünger, A.T. X-PLOR Version 3.1 A System for X-ray Crystallography and NMR (Yale University Press, New Haven, 1988).
Wüthrich, K., NMR of Proteins and Nucleic Acids (John Wiley & Sons, New York, 1986).
Diamond, R., Protein Sci. 1, 1279–1287 (1992).
Nilges, M., Kuszewski, J. & Brunger, A.T. Computational aspects of the study of biological macromolecules by NMR (Plenum Press, New York, 1991).
Laskowski, R.A., Rullmann, J.A., MacArthur, M.W., Kaptein, R. & Thornton, J.M., J. Biomol. NMR 8, 477–486 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wood, M., Sampoli Benitez, B. & Komives, E. Solution structure of the smallest cofactor-active fragment of thrombomodulin. Nat Struct Mol Biol 7, 200–204 (2000). https://doi.org/10.1038/73302
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/73302
- Springer Nature America, Inc.
This article is cited by
-
Metabolic15N labeling of the N-glycosylated immunoglobulin G1 Fc with an engineered Saccharomyces cerevisiae strain
Journal of Biomolecular NMR (2022)
-
Thrombomodulin and its role in inflammation
Seminars in Immunopathology (2012)
-
Recent advances in segmental isotope labeling of proteins: NMR applications to large proteins and glycoproteins
Journal of Biomolecular NMR (2010)
-
Isotope labeling of mammalian GPCRs in HEK293 cells and characterization of the C-terminus of bovine rhodopsin by high resolution liquid NMR spectroscopy
Journal of Biomolecular NMR (2008)