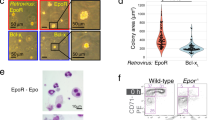
Abstract
MUTATIONS in the KIT transmembrane protein-tyrosine kinase receptor1 affect erythropoiesis, resulting in fewer committed late progenitors (colony-forming unit erythroid, CFU-E) in the fetal liver2. As the survival and proliferation of CFU-Es depend absolutely on erythropoietin (EPO)3, these results suggest that CFU-Es cannot proliferate or mature further unless both the KIT and EPO receptor4 signalling pathways are functional. How KIT affects proliferation or differentiation of CFU-Es is not clear. Here we show that the KIT ligand SCF (for stem-cell factor) can replace EPO in supporting the growth and survival of HCD57 cells, an EPO-dependent erythroid-progenitor cell line expressing high levels of KIT5. SCF supports the proliferation of 32D cells6 that express KIT only if they also express the EPO receptor. In HCD57 cells, SCF rapidly induces tyrosine phosphorylation of the EPO receptor, and KIT physically associates with the extended box 2 region7 in the cytoplasmic domain of the EPO receptor. Our results indi-cate that KIT may activate the EPO receptor by tyrosine phosphorylation to induce further proliferation and maturation of CFU-Es.
Similar content being viewed by others
References
Qiu, F. et al. EMBO J. 7, 1003–1011 (1988).
Nocka, K. et al. Genes Dev. 3, 816–826 (1989).
Gregory, C. L. J. Cell Physiol. 89, 289–302 (1976).
D'Andrea, A. D., Lodish, H. F. & Wong, G. G. Cell 57, 277–285 (1989).
Spivak, J. L. Pham, T., Isaacs, M. & Hankins, W. D. Blood 77, 1228–1233 (1991).
Hilton, D. J. et al. Proc. natn. Acad. Sci. U.S.A. 92, 190–194 (1995).
He, T. C. et al. J. biol. Chem. 269, 18291–18294 (1994).
Liu, L., Cutler, R. L., Mui, A. L. F. & Krystal, G. J. biol. Chem. 269, 16774–16779 (1994).
Serve, H. et al. EMBO J. 14, 473–483 (1995).
Klingmüller, U., Lorenz, U., Cantley, L. C., Neel, B. G. & Lodish, H. F. Cell 80, 729–738 (1995).
Rottapel, R. et al. Molec. cell. Biol. 11, 3043–3051 (1991).
Tsujimura, T. et al. Blood 83, 2619–2626 (1994).
Yoshimura, A., D'Andrea, A. D. & Lodish, H. F. Proc. natn. Acad. Sci. U.S.A. 87, 4139–4143 (1990).
Letwin, K., Yee, S. P. & Pawson, T. Oncogene 3, 621–627 (1988).
Reedijk, M. et al. EMBO J. 11, 1365–1372 (1992).
Yoshimura, A. & Lodish, H. F. Molec. cell. Biol. 12, 706–715 (1992).
Witthuhn, B. A. et al. Cell 74, 227–236 (1993).
Miura, Y., Miura, O., Ihle, J. N. & Aoki, N. J. biol. Chem. 269, 29962–29996 (1994).
Besmer, P. Curr. Opin. Cell Biol. 3, 939–946 (1991).
Majumder, S., Brown, K., Qiu, F. & Besmer, P. Molec. cell. Biol. 8, 4896–4903 (1988).
Sawyer, S. T. & Hankins, W. D. Proc. natn. Acad. Sci. U.S.A. 90, 6849–6853 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wu, H., Klingmüller, U., Besmer, P. et al. Interaction of the erythropoietin and stem-cell-factor receptors. Nature 377, 242–246 (1995). https://doi.org/10.1038/377242a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/377242a0
- Springer Nature Limited
This article is cited by
-
Ferric citrate and apo-transferrin enable erythroblast maturation with β-globin from hemogenic endothelium
npj Regenerative Medicine (2023)
-
Why Are Periodic Erythrocytic Diseases so Rare in Humans?
Bulletin of Mathematical Biology (2022)
-
Ex vivo-Expansion von roten Blutzellen aus humanen Stammzellen
BIOspektrum (2020)
-
Macrophages support pathological erythropoiesis in polycythemia vera and β-thalassemia
Nature Medicine (2013)
-
Skeletal muscle alterations and exercise performance decrease in erythropoietin-deficient mice: a comparative study
BMC Medical Genomics (2012)