Abstract
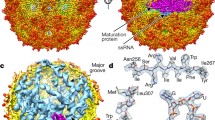
THE RNA bacteriophage MS2 is a convenient model system for the study of protein–RNA interactions. The MS2 coat protein achieves control of two distinct processes—sequence-specific RNA encapsidation and repression of replicase translation—by binding to an RNA stem–loop structure of 19 nucleotides containing the initiation codon of the replicase gene. The binding of a coat protein dimer to this hairpin shuts off synthesis of the viral replicase1, switching the viral replication cycle to virion assembly rather than continued replication. The operator fragment alone can trigger self-assembly of the phage capsid at low protein concentrations and a complex of about 90 RNA operator fragments per protein capsid has been described2. We report here the crystal structure at 3.0 Å resolution of a complex between recombinant MS2 cap-sids and the 19-nucleotide RNA fragment. It is the first example of a structure at this resolution for a sequence-specific protein-RNA complex apart from the transfer RNA synthetase complexes3–5. The structure shows sequence-specific interactions between conserved residues on the protein and RNA bases essential for binding.
Similar content being viewed by others
References
Witherell, G. W., Gott, J. M. & Uhlenbeck, O. C. Progr. Nucleic. Acid Res. molec. Biol. 40, 185–220 (1991).
Beckett, D. & Uhlenbeck, O. C. J. molec. Biol. 204, 927–938 (1988).
Rould, M. A., Perona, J. J. & Steitz, T. A. Nature 352, 213–218 (1991).
Ruff, M. et al. Science 252, 1682–1689 (1991).
Cavarelli, J., Rees, B., Ruff, M., Thierry, J.-C. & Moras, D. Nature 362, 181–184 (1993).
Valegård, K., Liljas, L., Fridborg, K. & Unge, T. Nature 345, 36–41 (1990).
Romaniuk, P. J., Lowary, P. T., Wu, H. N., Stormo, G. & Uhlenbeck, O. C. Biochemistry 26, 1563–1568 (1987).
Talbot, S. J., Goodman, S., Bates, S. R. E., Fishwick, C. W. G. & Stockley, P. G. Nucleic Acids Res. 18, 3521–3528 (1990).
Peabody, D. S. EMB0 J. 12, 595–600 (1993).
Stockley, P. G. et al. Biochem. Soc. Trans. 21, 627–633 (1993).
Peabody, D. S. Nucleic Acids Res. 17, 6017–6027 (1989).
Lim, F., Spingola, M. & Peabody, D. S. J. biol. Chem. 269, 9006–9010 (1994).
Harrison, S. C., Olson, A. J., Schutt, C. E., Winkler, F. K. & Bricogne, G. Nature 276, 368–373 (1978).
Abad-Zapatero, C. et al. Nature 286, 33–39 (1980).
Hogle, J. M., Maeda, A. & Harrison, S. C. J. molec. Biol. 191, 625–638 (1986).
Hosur, M. V. et al. Prot. Struct. Fund. Genet. 2, 167–176 (1987).
Liddington, R. et al. Nature 354, 278–284 (1991).
Sorger, P. K., Stockley, P. G. & Harrison, S. C. J. molec. Biol. 191, 639–658 (1986).
Goimohammadi, R., Valegård, K., Fridborg, K. & Liljas, L. J. molec. Biol. 234, 620–639 (1993).
Beckett, D., Wu, H.-N. & Uhlenbeck, O. C. J. molec. Biol. 204, 939–947 (1988).
Mastico, R. A., Talbot, S. J. & Stockley, P. G. J. gen. Virol. 74, 541–542 (1993).
Usman, N., Ogilvie, K. K., Tiang, M.-Y. & Cedergren, R. G. J. Am. chem. Soc. 109, 7845–7854 (1987).
Murray, J. B., Collier, A. K. & Arnold, J. R. P. Analyt. Biochem. 218, 177–184 (1994).
Jones, T. A. in CCP4 Study Weekend 1992; Molecular Replacement (eds, Dodson, E. J., Gover, S. & Wolf, W.) 91–105 (Daresbury Laboratory, UK, 1992).
Jones, T. A., Zou, J.-Y., Cowan, S. W. & Kjeldgaard, M. Acta crystallogr. A47, 110–119 (1991).
Brünger, A. T. XPLOR Manual (Yale University, New Haven, CT, 1990).
Brünger, A. T. Nature 355, 472–473 (1992).
Kraulis, P. J. J. appl. Crystallogr. 24, 946–950 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Valegård, K., Murray, J., Stockley, P. et al. Crystal structure of an RNA bacteriophage coat protein–operator complex. Nature 371, 623–626 (1994). https://doi.org/10.1038/371623a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/371623a0
- Springer Nature Limited
This article is cited by
-
Automated design of protein-binding riboswitches for sensing human biomarkers in a cell-free expression system
Nature Communications (2023)
-
Optimizing the synthesis and purification of MS2 virus like particles
Scientific Reports (2021)
-
Production and characterization of novel ssRNA bacteriophage virus-like particles from metagenomic sequencing data
Journal of Nanobiotechnology (2019)
-
Intrinsically-disordered N-termini in human parechovirus 1 capsid proteins bind encapsidated RNA
Scientific Reports (2018)
-
Single-mRNA detection in living S. cerevisiae using a re-engineered MS2 system
Nature Protocols (2018)