Abstract
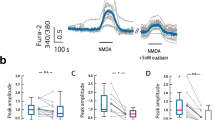
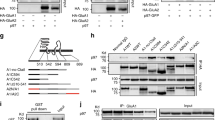
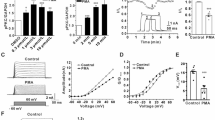
PHOSPHORYLATION of molecules involved in synaptic transmission by multifunctional protein kinases modulates both pre- and post-synaptic events in the central nervous system1,2. The positioning of kinases near their substrates may be an important part of the regulatory mechanism. The A-kinase-anchoring proteins (AKAPs; ref. 3) are known to bind the regulatory subunit of cyclic AMP-dependent protein kinase A with nanomolar affinity4–6. Here we show that anchoring of protein kinase A by AKAPs is required for the modulation of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA)/kainate channels4,5. Intracellular per-fusion of cultured hippocampal neurons with peptides derived from the conserved kinase binding region of AKAPs prevented the protein kinase A-mediated regulation of AMPA/kainate currents as well as fast excitatory synaptic currents. This effect could be overcome by adding the purified catalytic subunit of protein kinase. A control peptide lacking kinase-binding activity had no effect. To our knowledge, these results provide the first evidence that anchoring of protein kinase A is crucial in the regulation of synaptic function.
Similar content being viewed by others
References
Greengard, P., Valtorta, F., Czernik, A. J. & Benfenati, F. Science 259, 780–784 (1993).
Levitan, I. A. Rev. Neurosci. 11, 119–136 (1988).
Carr, D. W., Stofko-Hahn, R. E., Fraser, I. D. C., Cone, R. D. & Scott, J. D. J. biol. Chem. 267, 16816–16823 (1992).
Bregman, D. B., Bhattacharyya, N. & Rubin, C. S. J. biol. Chem. 264, 4648–4656 (1989).
Carr, D. W. et al. J. biol. Chem. 266, 14188–14192 (1991).
Carr, D. W., Hausken, Z. E., Fraser, I. D. C., Stofko-Hahn, R. E. & Scott, J. D. J. biol. Chem. 267, 13376–13382 (1992).
Wang, L.-Y., Salter, M. W. & MacDonald, J. F. Science 253, 1132–1135 (1991).
Greengard, P., Jen, J., Nairn, A. C. & Stevens, C. F. Science 253, 1135–1138 (1991).
Swope, S., Moss, S. J., Blackstone, C. D. & Huganir, R. L. FASEB 6, 2514–2523 (1992).
Uhler, M. D., Chrivia, J. C. & McKnight, G. S. J. biol. Chem. 261, 15360–15363 (1986).
Hofmann, F., Beavo, J. A., Bechtel, P. J. & Krebs, E. G. J. biol. Chem. 250, 7795–7801 (1975).
Barsony, J. & Marks, S. J. Proc. natn. Acad. Sci. U.S.A. 87, 1188–1192 (1990).
Bacskai, B. J. et al. Science 260, 222–226 (1993).
Rubin, C. S., Rangel-Aldao, R., Sarkar, D., Ehrlichman, J. & Fleischer, N. J. biol. Chem. 254, 3797–3805 (1979).
Corbin, J. D., Keely, S. L. & Park, C. R. J. biol. Chem. 250, 218–225 (1975).
Patneau, D. K. & Mayer, M. L. J. Neurosci. 10, 2385–2399 (1990).
Gasic, G. P. & Hollmann, M. A. Rev Physiol. 54, 507–536 (1992).
Sommer, B. & Seeburg, P. Trends pharmac. Sci. 13, 291–296 (1992).
Egebjerg, J., Bettler, B., Hermans-Borgmeyer, I. & Heinemann, S. Nature 351, 745–748 (1991).
Wang, L.-Y., Taverna, F. A., Huang, X.-P., MacDonald, J. F. & Hampson, D. R. Science 259, 1173–1175 (1993).
Raymond, L. A., Blackstone, C. D. & Huganir, R. L. Nature 361, 637–641 (1993).
Keller, B. U., Hollmann, M., Heinemann, S. & Konnerth, A. EMBO. J. 11, 891–896 (1992).
Chavez-Noriega, L. E. & Stevens, C. F. Brain Res. 574, 85–92 (1992).
Threurfauf, W. & Vallee, R. J. biol. Chem. 257, 3284–3290 (1982).
De Camilli, P., Moretti, M., Donini, S. D., Walter, U. & Lohmann, S. M. J. Cell Biol. 103, 189–203 (1986).
Glantz, S. B., Amat, J. A. & Rubin, C. S. Molec. Biol. Cell 3, 1215–1228 (1992).
Hubbard, M. J. & Cohen, P. Trends biochem. Sci. 18, 172–177 (1993).
Legendre, P. & Westbrook, G. L. J. Physiol., Lond. 429, 429–449 (1990).
Rosenmund, C. & Westbrook, G. L. J. Physiol., Lond. 470, 705–729 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rosenmund, C., Carr, D., Bergeson, S. et al. Anchoring of protein kinase A is required for modulation of AMPA/kainate receptors on hippocampal neurons. Nature 368, 853–856 (1994). https://doi.org/10.1038/368853a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/368853a0
- Springer Nature Limited
This article is cited by
-
σ-1 Receptor Inhibition of ASIC1a Channels is Dependent on a Pertussis Toxin-Sensitive G-Protein and an AKAP150/Calcineurin Complex
Neurochemical Research (2015)
-
Heart failure-specific changes in protein kinase signalling
Pflügers Archiv - European Journal of Physiology (2014)