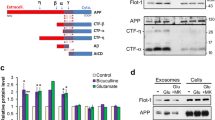
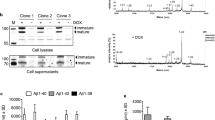
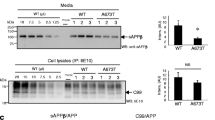
Abstract
THE accumulation in brain of senile plaques containing β-amyloid protein (Aβ) is a defining feature of Alzheimer's disease1–3. The amyloid precursor protein (APP)4 from which Aβ is derived is subject to several genetic mutations which segregate with rare familial forms of the disease, resulting in early onset of dementia and plaque formation5–9, suggesting that APP metabolism plays a causal role in the disease. Various cell types have been shown to release a soluble form of Aβ, thus allowing for the in vitro study of Aβ generation10–12. We report here evidence that a substantial portion of the APP secreted by human mixed brain cell cultures, as well as that present in cerebrospinal fluid, is of a novel form cleaved precisely at the amino terminus of Aβ, suggesting that a secretory pathway is involved in Aβ genesis.
Similar content being viewed by others
References
Glenner, G. G. & Wong, C. W. Biochem. biophys. Res. Commun., 120, 885–890 (1984).
Masters, C. L. et al. Proc. natn. Acad. Sci. U.S.A. 82, 4245–4249 (1985).
Wong, C. W., Quaranta, V. & Glenner, G. G. Proc. natn. Acad Sci. U.S.A. 82, 8729–8732 (1985).
Kang, J. et al. Nature 325, 733–736 (1987).
Goate, A. et al. Nature 349, 704–706 (1991).
Murrell, J., Farlow, M., Ghetti, B. & Benson, M. Science 254, 97–99 (1991).
Chartier-Harlin, M. C. et al. Nature 353, 844–846 (1991).
Hendricks, L. et al. Nature Genet. 1, 218–221 (1992).
Mullan, M. et al. Nature Genet. 1, 345–347 (1992).
Seubert, P. et al. Nature 359, 325–327 (1992).
Haass, C. et al. Nature 359, 322–325 (1992).
Shoji, M. et al. Science 258, 126–129 (1992).
Sisodia, S. S., Koo, E. H., Beyreuther, K., Unterbeck, A. & Price, O. L. Science 248, 492–495 (1990).
Esch, F. S. et al. Science 248, 1122–1124 (1990).
Anderson, J. P. et al. Neurosci. Lett. 128, 126–128 (1991).
Ponte, P. et al. Nature 331, 525–527 (1988).
Tanzi, R. E. et al. Nature 331, 528–530 (1988).
Weidemann, A. et al. Cell 57, 115–126 (1989).
Selkoe, D. J. et al. Proc. natn. Acad. Sci. U.S.A. 85, 7341–7345 (1988).
Golde, T. E., Estus, S., Younkin, I. H., Selkoe, D. J. & Younkin, S. G. Science 255, 728–730 (1992).
Hyman, B. T., Tanzi, R. E., Marzloff, B. A., Barbour, R. & Schenk, D. J. Neuropath. exp. Neurol. 51, 76–83 (1992).
Kennedy, H., Kametani, F. & Allsop, D. Neurodegeneration 1, 59–64 (1992).
Anderson, J. P., Chen, U., Kim, K. S. & Robakis, N. K. J. Neurochem. 59, 2328–2331 (1992).
Oltersdorf, T. et al. Nature 341, 144–147 (1989).
Pulliam, L., Dix, R. D., Panitch, H. S. & Baringer, J. R. J. virol. Meth. 9, 301–306 (1984).
Oltersdorf, T. et al. J. biol. Chem. 265, 4492–4497 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Seubert, P., Oltersdorf, T., Lee, M. et al. Secretion of β-amyloid precursor protein cleaved at the amino terminus of the β-amyloid peptide. Nature 361, 260–263 (1993). https://doi.org/10.1038/361260a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/361260a0
- Springer Nature Limited
This article is cited by
-
Cholesterol Metabolism in Neurodegenerative Diseases: Molecular Mechanisms and Therapeutic Targets
Molecular Neurobiology (2021)
-
A method of predicting the in vitro fibril formation propensity of Aβ40 mutants based on their inclusion body levels in E. coli
Scientific Reports (2019)
-
Expression analysis of beta-secretase 1 (BACE1) and its naturally occurring antisense (BACE1-AS) in blood of epileptic patients
Neurological Sciences (2018)
-
MMP-7 cleaves amyloid β fragment peptides and copper ion inhibits the degradation
BioMetals (2017)
-
Redundant Gs-coupled serotonin receptors regulate amyloid-β metabolism in vivo
Molecular Neurodegeneration (2016)