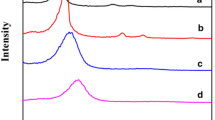
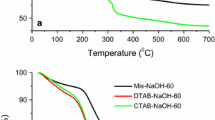
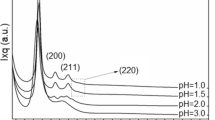
Abstract
MICROPOROUS and mesoporous inorganic solids (with pore diameters of ≤20 Å and ∼20–500 Å respectively)1 have found great utility as catalysts and sorption media because of their large internal surface area. Typical microporous materials are the crystalline framework solids, such as zeolites2, but the largest pore dimensions found so far are ∼10–12 Å for some metallophosphates3–5 and ∼14 Å for the mineral cacoxenite6. Examples of mesoporous solids include silicas7 and modified layered materials8–11, but these are invariably amorphous or paracrystalline, with pores that are irregularly spaced and broadly distributed in size8,12. Pore size can be controlled by intercalation of layered silicates with a surfactant species9,13, but the final product retains, in part, the layered nature of the precursor material. Here we report the synthesis of mesoporous solids from the calcination of aluminosilicate gels in the presence of surfactants. The material14,15 possesses regular arrays of uniform channels, the dimensions of which can be tailored (in the range 16 Å to 100 Å or more) through the choice of surfactant, auxiliary chemicals and reaction conditions. We propose that the formation of these materials takes place by means of a liquid-crystal 'templating' mechanism, in which the silicate material forms inorganic walls between ordered surfactant micelles.
Similar content being viewed by others
References
IUPAC Manual of Symbols and Terminology Pure appl. Chem. 31, 578 (1972).
Meier, W. M. & Olson, D. H. Atlas of Zeolite Structure Types, 2nd Edn (Butterworths, London, 1988).
Davis, M. E., Saldarriaga, C., Montes, C., Garces, J. & Crowder, C. Nature 331, 698–699 (1988).
Dessau, R. M., Schlenkar, J. L. & Higgins, J. B. Zeolites 10, 522–524 (1990).
Estermann, M., McCusker, L. B., Baerlocher, C., Merrouche, A. & Kessler, H. Nature 352, 320–323 (1991).
Moore, P. B. & Shen, J. Nature 306, 356–358 (1983).
Iler, R. K. The Chemistry of Silica (Wiley, New York, 1979).
Pinnavaia, T. J. Science 220, 365–371 (1983).
Landis, M. E. et al. J. Am. chem. Soc. 113, 3189–3190, 1991.
Vaughan, D. E. W. & Lussier, R. J. Proc. 5th int. Conf. Zeolites (ed. Rees, L. V. C.) 94–100 (Heyden, London, 1980).
Vaughan, D. E. W. Am. chem. Soc. Symp. Series 368, 308–325 (1988).
Tindwa, R. M., Ellis, D. K., Peng, G. Z. & Clearfield, A. J. chem. Soc. Faraday Trans. 1, 81, 545–548 (1985).
Yanagisawa, T., Shimizu, T., Kazuyuki, K. & Kato, C. Bull. Chem. Soc. Jpn 63, 988–992 (1990).
Kresge, C. T., Leonowicz, M. E., Roth, W. J. & Vartuli, J. C. U.S. Patent No. 5,098,684 (1992); U.S. Patent No. 5,102,643 (1992).
Beck, J. S. et al. U.S. Patent No. 5,108,725 (1992).
Beck, J. S. U.S. Patent No. 5,057,296 (1991).
Ekwall, P. Advances in Liquid Crystals, Vol. 1 (ed. Brown, G. H.) (Academic, New York, 1971).
Ekwall, P., Mandell, L. & Fontell, K. Liquid Crystals (ed. Brown, G. H.) 325–334 (Gordon and Breach, London, 1969).
Luzzati, V. Biological Membranes (ed. Chapman, D.) 71–123 (Academic, New York, 1968).
Tiddy, G. J. T. Phys. Rep. 57, No. 1, 1–46 (1980).
Winsor, P. A. Chem. Rev. 68, No. 1, 1–40 (1968).
Goodman, J. F. & Clunie, J. S. Electron Microscopy of Liquid Crystals, Liquid Crystals and Plastic Crystals, Vol. 2 (eds Gray, G. W. & Winsor, P. A.) 1–23 (Wiley, New York, 1974).
Speght, P. P. A., Skoulios, A. E. & Luzzati, V. Acta cryst. 14, 866–872 (1961).
Komarov, V. S. & Kuznetsova, T. F. Vesti Akad. Navuk BSSR No. 2, 22–27 (1978).
Luzzati, V. & Speght, P. P. A. Nature 215, 701–704 1967.
Gregg, S. J. & Sing, K. S. W. Adsorption, Surface Area, and Porosity, 2nd Edn (Academic, New York, 1982).
Barrett, E. P., Joyner, L. G. & Halenda, P. P. J. Am. chem. Soc. 73, 373–380 (1951).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kresge, C., Leonowicz, M., Roth, W. et al. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 359, 710–712 (1992). https://doi.org/10.1038/359710a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/359710a0
- Springer Nature Limited
This article is cited by
-
Mesoporous silica coated spicules for photodynamic therapy of metastatic melanoma
Journal of Nanobiotechnology (2024)
-
Recent Advances Ultra-Porous Drug Nano-Carriers: Synthesis and Targeting Approaches
Silicon (2024)
-
Catalytic oxidation of lignin model non-phenolic monomer Veratryl alcohol over iron containing mesoporous SBA-1
Journal of Porous Materials (2024)
-
Ordered mesoporous MCM-41 containing cobalt ferrite as high-performance catalyst in the synthesis of 5-substituted 1H-tetrazoles and oxidation of sulfides
Journal of Porous Materials (2024)
-
Preparation of Co3O4 Nanoparticles in a Lyotropic Liquid Crystal Medium and Their Application in Anti-bacterial, Anti-cancer and Catalytic Activities
BioNanoScience (2024)