Abstract
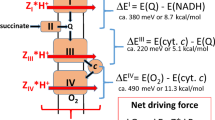
IN mitochondria, electrons derived from the oxidation of succinate by the tricarboxylic acid cycle enzyme succinate-ubiquinone oxido-reductase are transferred directly to the quinone pool. Here we provide evidence that the soluble form of this enzyme (succinate dehydrogenase) behaves as a diode that essentially allows electron flow in one direction only. The gating effect is observed when electrons are exchanged rapidly and directly between fully active succinate dehydrogenase and a graphite electrode. Turnover is therefore measured under conditions of continuously variable electrochemical potential. The otherwise rapid and efficient reduction of fumarate (the reverse reaction) is severely retarded as the driving force (overpotential) is increased. Such behaviour can arise if a rate-limiting chemical step like substrate binding or product release depends on the oxidation state of a redox group on the enzyme. The observation provides, for a biological electron-transport system, a simple demonstration of directionality that is enforced by kinetics as opposed to that which is assumed from thermodynamics.
Similar content being viewed by others
References
Ackrell, B. A. C., Johnson, M. K., Gunsalus, R. P. & Cecchini, G. in Chemistry and Biochemistry of Flavoenzymes Vol. 3 (ed. Mueller, F.) 229–297 (CRC, Boca Raton, Florida, 1992).
Davis, K. A. & Hatefi, Y. Biochemistry 10, 2509–2516 (1971).
Beinert, H., Ackrell, B. A. C., Vinogradov, A. D., Kearney, E. B. & Singer, T. P. Archs Biochem. Biophys. 182, 95–106 (1977).
Armstrong, F. A. Struct. Bond. 72, 137–221 (1990).
Bard, A. J. & Faulkner, L. R. Electrochemical Methods Ch. 8 (Wiley, New York, 1980).
Singer, T. P., Kearney, E. B. & Kenney, W. C. Adv. Enzym. 37, 189–272 (1973).
Ohnishi, T. et al. J. biol. Chem. 256, 5577–5582 (1981).
Collman, J. P. & Kim, K. J. Am. chem. Soc. 108, 7847–7849 (1986).
Zeijlemaker, W. P., Dervartanian, D. V., Veeger, C. & Slater, E. C. Biochlm. biophys. Acta 178, 213–224 (1968).
Mackenroth, D. R. & Sands, L. G. Illustrated Encylcopaedia of Solid-state Circuits and Applications Ch. 1 (Prentice-Hall, Englewood Cliffs, New Jersey, 1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sucheta, A., Ackrell, B., Cochran, B. et al. Diode-like behaviour of a mitochondrial electron-transport enzyme. Nature 356, 361–362 (1992). https://doi.org/10.1038/356361a0
Issue Date:
DOI: https://doi.org/10.1038/356361a0
- Springer Nature Limited
This article is cited by
-
Denitrification mechanism in oxygen-rich aquatic environments through long-distance electron transfer
npj Clean Water (2022)
-
Reversible catalysis
Nature Reviews Chemistry (2021)
-
The assembly of succinate dehydrogenase: a key enzyme in bioenergetics
Cellular and Molecular Life Sciences (2019)
-
Electroactive porous films of myoglobin within calcium alginate
Journal of Solid State Electrochemistry (2012)
-
Electron paramagnetic resonance and Mössbauer spectroscopy of intact mitochondria from respiring Saccharomyces cerevisiae
JBIC Journal of Biological Inorganic Chemistry (2007)