Abstract
COMMON rock-and soil-forming minerals are complicated structures of varying composition. Despite some encouraging progress1,2 there is as yet no comprehensive rationale for predicting the dissolution rates of these minerals. Here we test the hypothesis3 that dissolution rates of compositionally distinct orthosilicate minerals scale in a fashion similar to rates of water exchange around the corresponding dissolved, divalent cation. Although dissolution rates span several orders of magnitude, the hypothesis is sustained. Minerals containing alkaline-earth cations dissolve at rates that correlate with ionic size, whereas minerals containing first-row transition metals dissolve at rates that vary with the number of cationd-electrons. Both types of behaviour are consistent with the control of dissolution rate by the character of the bonds between the divalent cation and neighbouring oxygen atoms. This result supports the proposed link3–6 between the mechanisms of mineral dissolution and the mechanisms by which a dissolved metal exchanges ligands. With this link it may be possible to predict dissolution rates for other nearly isostructural minerals that vary in composition.
Similar content being viewed by others
References
Brady, P. V. & Walther, J. V. Geochim. cosmochim. Acta 53, 2823–2830 (1989).
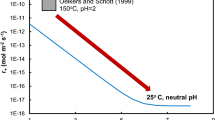
Schott, J., Berner, R. A. & Sjoberg, E. L. Geochim. cosmochim. acta 45, 2123–2135 (1981).
Casey, W. H. J. Colloid. Interface Sci. 135, (in the press).
Stumm, W. & Wollast, R. Rev. Geophys. 28, 53–69 (1990).
Burns, R. G. Mineralogical Applications of Crystal Field Theory (Cambridge University Press, London, 1970).
Casey, W. H., Lasaga, A. C. & Gibbs, G. V. Geochim. cosmochim. Acta 54, 3369–3378 (1990).
Bish, D. L. & Burnham, C. W. Am. Mineral. 69, 1102–1109 (1984).
Burgess, J. Ions in Solution (Wiley, New York, 1988).
Wilkens, R. G. The Study of Kinetics and Mechanisms of Reactions of Transition Metal Complexes (Allyn-Bacon, Boston, 1974).
Brown, G. E. in Orthosilicates (ed. Ribbe, P.) 175–365 (Mineralogical Society of America, Washing ton DC, 1983).
Terry, B. & Monhemius, A. J. Metall. Trans. B14, 335–326 (1983).
Blum, A. & Lasaga, A. C. Nature 331, 431–433 (1988).
Grandstaff, D. E. Proc. 3rd Int. Symp. Water-Rock Interaction, 72–74 (Alberta Research Council, Edmonton, 1980).
Wogelius, R. A. & Walther, J. V. Geochim. cosmochim. Acta 55, 943–954 (1991).
Hewkin, D. J. & Prince, R. H. Coord. Chem. Rev. 5, 45–73 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Casey, W., Westrich, H. Control of dissolution rates of orthosilicate minerals by divalent metal–oxygen bonds. Nature 355, 157–159 (1992). https://doi.org/10.1038/355157a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/355157a0
- Springer Nature Limited
This article is cited by
-
Crystal dissolution by particle detachment
Nature Communications (2023)
-
Replacement reactions for carbon geosequestration may be faster in calcium olivine vs magnesium olivine
Communications Earth & Environment (2023)
-
Molecular-scale mechanisms of CO2 mineralization in nanoscale interfacial water films
Nature Reviews Chemistry (2022)
-
Acidic condition accelerates cation release from purple rock in Southwestern China
Scientific Reports (2022)
-
Inhibitory Effect of MgO, FeO, CaF2, and Al2O3 Additives on the Dissolution Behavior of Ca from Silicate Mineral Phases into Water
Metallurgical and Materials Transactions B (2022)