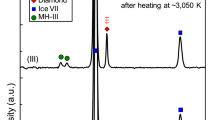
Abstract
Methane hydrate is thought to have been the dominant methane-containing phase in the nebula from which Saturn, Uranus, Neptune and their major moons formed1. It accordingly plays an important role in formation models of Titan, Saturn's largest moon. Current understanding1,2 assumes that methane hydrate dissociates into ice and free methane in the pressure range 1–2 GPa (10–20 kbar), consistent with some theoretical3 and experimental4,5 studies. But such pressure-induced dissociation would have led to the early loss of methane from Titan's interior to its atmosphere, where it would rapidly have been destroyed by photochemical processes6,7. This is difficult to reconcile with the observed presence of significant amounts of methane in Titan's present atmosphere. Here we report neutron and synchrotron X-ray diffraction studies that determine the thermodynamic behaviour of methane hydrate at pressures up to 10 GPa. We find structural transitions at about 1 and 2 GPa to new hydrate phases which remain stable to at least 10 GPa. This implies that the methane in the primordial core of Titan remained in stable hydrate phases throughout differentiation, eventually forming a layer of methane clathrate approximately 100 km thick within the ice mantle. This layer is a plausible source for the continuing replenishment of Titan's atmospheric methane.


Similar content being viewed by others
References
Lunine, J. I. & Stevenson, D. J. Clathrate and ammonia hydrates at high-pressure—application to the origin of methane on Titan. Icarus 70, 61–77 (1987).
Grasset, O., Sotin, C. & Deschamps, F. On the internal structure and dynamics of Titan. Planet. Space Sci. 48, 617–636 (2000).
Lunine, J. I. & Stevenson, D J. Thermodynamics of clathrate hydrate at low and high pressures with application to the outer solar system. Astrophys. J. Suppl. Ser. 58, 493–531 (1985).
Hirai, H. et al. Methane hydrate behaviour under high pressure. J. Phys. Chem. B 104, 1429–1433 (2000).
Hirai, H. et al. Methane hydrate, amoeba or a sponge made of water molecules. Chem. Phys. Lett. 325, 490–498 (2000).
Lorenz, R. D. The weather on Titan. Science 290, 467–468 (2000).
Lunine, J. I. Does Titan have an ocean? A review of current understanding of Titan's surface. Rev. Geophys. 31, 133–149 (1993).
Gutt, C. et al. The structure of deuterated methane hydrate. J. Chem. Phys. 113, 4713–4721 (2000).
van der Waals, J. H. Statistical mechanics of clathrate compounds. Trans. Faraday Soc. 52, 184 (1956).
van der Waals, J. H. & Platteeuw, J. C. Clathrate solutions. Adv. Chem. Phys. 2, 1 (1959).
Dyadin, Y. A., Aladko, E. Y. & Larionov, E. G. Decomposition of methane hydrates up to 15 kbar. Mendeleev Commun. 7, 34–35 (1997).
Pistorius, C. W., Pistorius, M. C., Blakey, J. P. & Adimiraal, L. J. Melting curve of ice VII to 200 kbar. J. Chem. Phys. 38, 600 (1963).
Udachin, K. A., Ratcliffe, C. I., Enright, G. D. & Ripmeester, J. A. Structure H Hydrate: a single crystal diffraction study of 2,2 dimethylpentane.5(Xe,H2S).34H2O. Supramol. Chem. 8, 173–176 (1997).
Chou, I. M. et al. Transformations in methane hydrates. Proc. Natl Acad. Sci. 97, 13484–13487 (2000).
Dyadin, Y. A. et al. Clathrate hydrates of hydrogen and neon. Mendeleev Commun. 5, 209–210 (1999).
Dyadin, Y. A., Larionov, E. G., Mirinski, D. S., Mikina, T. V. & Starostina, L. I. Clathrate formation in the Ar-H2O system under pressures up to 15,000 bar. Mendeleev Commun. 7, 32–34 (1997).
Lotz, H. T. & Schouten, J. A. Clathrate hydrates in the system H2O-Ar at pressures and temperatures up to 30 kbar and 140 °C. J. Chem. Phys. 111, 10242–10247 (1999).
van Hinsberg, M. G. E., Scheerboom, M. I. M. & Schouten, J. A. The vibrational spectra of N2 in clathrate-hydrates: A new high-pressure phase transition. J. Chem. Phys. 99, 752–754 (1993).
Grasset, O. & Sotin, C. The cooling rate of a liquid shell in Titan's interior. Icarus 123, 101–112 (1996).
Stevenson, D. J. in Proceedings of Symposium on Titan 29–33 (European Space Agency Special Publication SP-338, Toulouse, 1992).
Handa, Y. P. Compositions, enthalpies of dissociation, and heat-capacities in the range 85K–270K for clathrate hydrates of methane, ethane, and propane, and enthalpy of dissociation of isobutane hydrate, as determined by a heat-flow calorimeter. J. Chem. Therm. 18, 915–921 (1986).
Nelmes, R. J. et al. Structure studies at high pressure using neutron powder diffraction. Trans. Am. Cryst. Ass. 29, 19–27 (1993).
PEARL—Pressure and Engineering Research Line 28–29 ISIS'97 Rutherford Appleton Laboratory Report RAL-TR-97-050 (RAL, Didcot, 1997).
Nelmes, R. J. & McMahon, M. I. High-pressure powder diffraction on synchrotron sources. J. Synch. Rad. 1, 69–73 (1994).
Acknowledgements
We thank D. J. Francis, T. Bovornratanaraks and M. Hanfland for assistance with experiments, and O. Grasset and D. J. Stevenson for helpful discussions of their work. Our work is funded by a research grant from the EPSRC, and supported by CCLRC through access to beamtime and other resources.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loveday, J., Nelmes, R., Guthrie, M. et al. Stable methane hydrate above 2 GPa and the source of Titan's atmospheric methane. Nature 410, 661–663 (2001). https://doi.org/10.1038/35070513
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35070513
- Springer Nature Limited
This article is cited by
-
Significance of the high-pressure properties and structural evolution of gas hydrates for inferring the interior of icy bodies
Progress in Earth and Planetary Science (2023)
-
A post-Cassini view of Titan’s methane-based hydrologic cycle
Nature Geoscience (2018)
-
Elasticity and Stability of Clathrate Hydrate: Role of Guest Molecule Motions
Scientific Reports (2017)
-
Fast methane diffusion at the interface of two clathrate structures
Nature Communications (2017)
-
Microscopic Origin of Strain Hardening in Methane Hydrate
Scientific Reports (2016)