Abstract
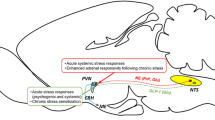
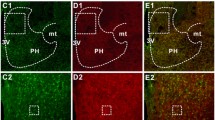
In response to stress, adrenocorticotropic hormone (ACTH) is released by corticotrophs in the anterior pituitary under the control of several central and peripheral factors1,2 including corticotropin-releasing factor (CRF), which was recently isolated from the brain and sequenced3. Immunocytochemical studies have shown that most of the CRF-containing cell bodies that project to the median eminence are present in the hypothalamic paraventricular nucleus (PVN)4–6. A dense PNMT(phenylethanolamine-N-methyltrans-ferase)-containing fibre network was also observed in the same region7,8—PNMT is the final enzyme in the biosynthesis of adrenaline9 and has been demonstrated in the brain10. In the present study we found an association of adrenergic nerve fibres and CRF neurones by immunohistochemistry using antisera to PNMT and CRF. To examine the functional significance of the adrenergic projection to the PVN, we blocked the synthesis of adrenaline using a specific inhibitor of PNMT. The depletion of adrenaline resulted in an increase in CRF immunoreactivity. The present results suggest that, as well as catecholamines which regulate ACTH release at the anterior pituitary level via a β2-adrenergic receptor mechanism9, central catecholamines (mainly adrenaline) also affect ACTH release through their action on CRF cells. Peripheral catecholamines seem to have a direct stimulatory effect on the pituitary corticotroph cells11, whereas the present findings suggest that central adrenaline-containing neurones have an inhibitory role in the physiological response to stress.
Similar content being viewed by others
References
Ganong, W. F. Brain-Endocrine Interaction: Median Eminence: Structure and Function (eds Knigge, J., Scott, D. S. & Weindl, A.) 254–266 (Karger, Basel, 1972).
Jones, M. T. The Endocrine Hypothalamus (eds Jeffcoate, S. L. & Hutchinson, J. S. H.) 386–419 (Academic, New York, 1978).
Vale, W., Spiess, J., Rivier, C. & Rivier, J. Science 213, 1394–1396 (1981).
Bloom, F. E., Battenberg, E. L. F., Rivier, J. & Vale, W. Regul. Peptides 4, 43–48 (1982).
Cummings, S., Elde, R., Ells, J. & Lindall, A. J. Neuroscience 3, 1355–1368 (1983).
Swanson, L. W., Sawchenko, P. E., Rivier, J. & Vale, W. Neuroendocrinology 36, 165–186 (1983).
Hokfelt, T., Fuxe, K., Goldstein, M. & Johansson, O. Brain Res. 66, 235–251 (1974).
Swanson, L. et al. J. comp. Neurol. 196, 271–285 (1981).
Axelrod, J. J. biol. Chem. 237, 1657–1660 (1962).
Saavedra, J., Palkovits, M., Brownstein, M. J. & Axelrod, J. Nature 248, 695–696 (1974).
Mezey, E., Reisine, T., Palkovits, M., Brownstein, M. J. & Axelrod, J. Proc. natn. Acad. Sci. U.S.A. 80, 6728–6731 (1983).
Ganong, W. F. Fedn Proc. 39, 2923–2930 (1980).
Weiner, R. & Ganong, W. F. Phys. Rev. 58, 905–976 (1978).
Roth, K. A. et al. Life Sci. 28, 2389–2394 (1981).
Turner, B. B., Katz, R. J., Roth, K. A. & Carroll, B. J. Brain Res. 153, 419–422 (1978).
Seybold, V., Elder, R. & Hokfelt, T. Endocrinology 108, 1803–1809 (1983).
Kiss, J. Z., Mezey, E. & Skirboll, L. Proc. natn. Acad. Sci. U.S.A. (in the press).
Swanson, L. W. & Hartman, B. K. J. comp. Neurol. 163, 467–506 (1975).
Fuller, R. W. et al. Biochem. Pharmac. 30, 1345–1352 (1981).
Sternberger, L. Immunocytochemistry (Wiley, New York, 1979).
Hsu Su-Ming & Ree, H. J. Am J. clin. Path. 74, 32–40 (1980).
Hsu Su-Ming, Raine, L. & Fanger, H. J. Histochem. Cytochem. 29, 577–580 (1981).
Inoue, S. & Sato, H. J. gen. Physiol. 50, 259–288 (1967).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mezey, É., Kiss, J., Skirboll, L. et al. Increase of corticotropin-releasing factor staining in rat paraventricular nucleus neurones by depletion of hypothalamic adrenaline. Nature 310, 140–141 (1984). https://doi.org/10.1038/310140a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/310140a0
- Springer Nature Limited
This article is cited by
-
Single-cell morphological characterization of CRH neurons throughout the whole mouse brain
BMC Biology (2021)
-
Effect of acute and chronic diisopropylfluorophosphate and atropine administration on somatostatin binding in the rat frontoparietal cortex and hippocampus
Psychopharmacology (1993)
-
Hypothesis: Cytokines may be activated to cause depressive illness and chronic fatigue syndrome
European Archives of Psychiatry and Clinical Neuroscience (1992)
-
Long-term depressor effects of catecholamine neuronal grafts in the third ventricle of the brain in normotensive rats
Experientia (1991)
-
Endocrine and neurochemical effects of (+)-PHNO, a dopamine D2 agonist
Journal of Neural Transmission (1989)