Abstract
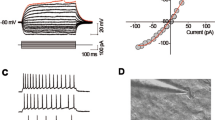
Ample evidence exists for histaminergic1 and noradrenergic2,3 projections to the hippocampus. Both amines exert neurotransmitter or modulator actions on principal neurones in the CA 1 and in the dentate area. A number of mechanisms have been proposed for these actions, including increased potassium conductance4, increased chloride conductance and electrogenic pump stimulation5,6, and reduction of the anomalous inward rectification5. Action potentials, and particularly bursts of spikes, in CA 1 pyramidal cells, are followed by an afterhyper-polarization (AHP) which consists of two components7. The late AHP depends on a calcium-activated potassium conductance gK+ (Ca2+)8–13, and has recently been shown to be increased by dopamine14. We report here a rapid and reversible decrease of the late AHP component following a burst of sodium spikes or a calcium spike, during perfusion with micromotor concentrations of histamine and noradrenaline2. This effect is mediated by H2 receptors and β-receptors, respectively, and occurred in the absence of changes in the calcium spike. By such a mechanism histamine and noradrenaline can profoundly potentiate the excitatory impact of depolarizing signals.
Similar content being viewed by others
References
Schwartz, J. C. A. Rev. Pharmac. Tox. 17, 325–339 (1974).
Ungerstedt, U. Actaphysiol. scand., Suppl. 367, 1–48 (1971).
Segal, M. & Bloom, F. E. Brain Res. 72, 99–113 (1974).
Haas, H. L. Neurosci. Lett. 22, 75–78 (1981).
Langmoen, I. A., Segal, M. & Andersen, P. Brain Res. 208, 349–362 (1981).
Segal, M. Brain Res. 206, 107–128 (1981).
Gustafsson, B. & Wigstroem, H. Brain Res. 206, 462–468 (1981).
Alger, B. E. & Nicoll, R. A. Science 210, 1122–1124 (1980).
Heyer, C. B. & Lux, H. D. J. Physiol., Lond. 262, 349–382 (1976).
Hotson, J. R. & Prince, D. A. J. Neurophysiol. 43, 409–419 (1980).
Meech, R. W. A. Rev. Biophys. Bioengng 7, 1–18 (1978).
Meech, R. W. & Standen, N. B. J. Physiol., Lond. 249, 211–239 (1975).
Schwartzkroin, P. A. & Stafstrom, C. E. Science 210, 1125–1127 (1980).
Benardo, L. S. & Prince, D. A. Nature 297, 76–79 (1982).
Haas, H. L., Schaerer, B. & Vosmansky, M. J. Neurosci. Meth. 1, 323–325 (1979).
Schwartzkroin, P. A. & Slawsky, M. Brain Res. 135, 157–161 (1977).
Haas, H. L. & Gaehwiler, B.H. Neurosci. Lett. 19, 89–92 (1980).
Alger, B. E. & Nicoll, R. A. Brain Res. 200, 195–200 (1980).
Haas, H. L. & Rose, G. J. Physiol., Lond. 329, 541–552 (1982).
Segal, M. Neurosci. Lett. 19, 67–71 (1980).
Cottrell, G. A. Nature 296, 87–89 (1982).
Frederickson, R. C. A., Jordan, L. M. & Phillis, J. W. Brain Res. 35, 556–560 (1971).
Haas, H. L. & Wolf, P. Brain Res. 122, 269–279 (1977).
Krnjević, K., Lamour, Y., MacDonald, J. F. & Nistri, A. Can. J. Physiol. Pharmac. 56, 896–900 (1978).
Mueller, A. L., Hoffer, B. J. & Dunwiddie, T. V. Brain Res. 214, 113–126 (1981).
Madison, D. V. & Nicoll, R. A. Nature 299, 636–638 (1982).
Haas, H. L., Wolf, P., Palacios, J. M., Garbarg, M., Barbin, G. & Schwartz, J. C. Brain Res. 156, 275–291 (1978).
Palmer, G. C., Sulser, F. & Robison, G. A. Neuropharmacology 12, 327–337 (1973).
Segal, M., Greenberger, V. & Hofstein, R. Brain Res. 213, 351–364 (1981).
Hotson, J. R., Prince, D. A. & Schwartzkroin, P. A. J. Neurophysiol. 42, 889–895 (1979).
Woodward, D. J., Moises, H. C., Waterhouse, B. D., Hoffer, B. J. & Freedman, R. Fedn Proc. 38, 2109–2116 (1979).
Rogawski, M. A. & Aghajanian, G. K. Nature 287, 731–734 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Haas, H., Konnerth, A. Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. Nature 302, 432–434 (1983). https://doi.org/10.1038/302432a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/302432a0
- Springer Nature Limited
This article is cited by
-
Exposure to lead and its effect on sleep quality and digestive problems in soldering workers
Environmental Monitoring and Assessment (2019)
-
Selective Modulation of Histaminergic Inputs on Projection Neurons of Cerebellum Rapidly Promotes Motor Coordination via HCN Channels
Molecular Neurobiology (2016)
-
Histamine Receptor Expression, Hippocampal Plasticity and Ammonia in Histidine Decarboxylase Knockout Mice
Cellular and Molecular Neurobiology (2012)
-
The waking brain: an update
Cellular and Molecular Life Sciences (2011)