Abstract
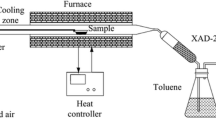
The release of 2,4,5-trichlorophenol (2,4,5-T) containing 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) at Sèveso1 highlighted the need for an efficient control procedure for the highly toxic2 dioxin. The concomitant formation of the dioxin and the trichlorophenol during hydrolysis of sym-tetra-chlorobenzene has lead to demands for restrictions on the use of 2,4,5-T and related herbicides3. An environmental hazard is also presented by pentachlorophenol4 which includes the more highly chlorinated dioxins, in particular octachlorodibenzo-p-dioxin (OCDD). The use of pholychlorophenol formulations has been banned in Sweden on the grounds5 that heat-stable chlorodioxins are formed during incineration; this also points to the need for degradation by chemical means. Dehalogenation of polychlorodibenzodioxins (PCDD) can be accomplished by photolysis in laboratory conditions6, but material adsorbed on soil is little affected7. Cleavage of the ether linkages with the formation of halophenols may be achieved by treatment with strong acids or quaternary ammonium salts8. The dibenzodioxin nucleus, is however, rather resistant to chemical attack9 and in the absence of a suitable control procedure the use of 2,4,5-T has been restricted in the US by the Environmental Protection Agency10,11. I now report the oxidative degradation of TCDD and related compounds by ruthenium tetroxide.
Similar content being viewed by others
References
Hay, A. Nature 281, 521 (1979).
Int. Agency Res. Cancer 15, 42 (1977).
Crosland, J. Ecologist 10, 87 (1980).
Dickens, D. Nature 283, 418 (1980).
Jansson, B. & Sundström, G. Sci. Total Envir. 10, 209 (1978).
Dobbs, A. J. & Grant, C. Nature 278, 163 (1979).
Kearney, P. C., Woolson, E. A. & Ellington, C. P. Envir. Sci. Technol. 6, 1017 (1972).
Botré, C., Memoli, A. & Alhaique, F. Envir. Sci. Technol. 13, 228 (1979).
Blair, E. H. (ed.) Chlorodioxins-Origin and Fate Adv. Chem. Ser., No. 120 (American Chemical Society, 1973).
Cookson, C. Nature 278, 108 (1979).
Josephson, J. Envir. Sci. Technol. 14, 1165 (1980).
Lee, D. G. & van den Engh, M. in Oxidation in Organic Chemistry (ed. Trahanovsky, W. S.) (Academic, New York, 1973).
Ayres, D. C. & Scott, C. M. Envir. Sci. Technol. 13, 1983 (1979).
Shine, H. J. & Shade, L. R. J. heterocycl. Chem. 11, 139 (1974).
Ayres, D. C. & Gopalan, R. JCS Chem. Commun. 890 (1976).
Mehendale, H. M., Fields, M. & Matthews, H. B. J. Agric. Food. Chem. 23, 261 (1975).
Ayres, D. C. & Levy, D. P. (in preparation).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ayres, D. Destruction of polychlorodibenzo-p-dioxins. Nature 290, 323–324 (1981). https://doi.org/10.1038/290323a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/290323a0
- Springer Nature Limited