Abstract
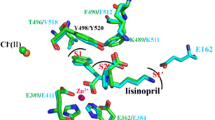
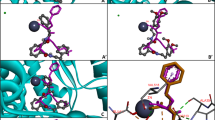
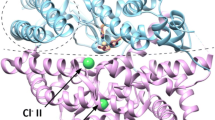
Much current attention focuses on the renin–angiotensin system in relation to mechanisms controlling blood pressure and renal function. Recent demonstrations (ref 1, ref. 2 and refs therein) that angiotensin-converting enzyme inhibitors show promising clinical antihypertensive properties have been of particular interest. We now report on the design of a novel series of substituted N-carboxymethyl-dipeptides which are active in inhibiting angiotensin-converting enzyme at nanomolar levels. We suggest that these compounds are transition-state inhibitors and that extensions of this design to other metalloendopeptidases merit further study.
Similar content being viewed by others
References
Gavras, H. et al. New Engl. J. Med. 291, 817–821 (1974).
Atkinson, A. B. & Robertson, J. I. S. Lancet ii, 836–839 (1979).
Skeggs, L. T., Marsh, W. H., Kahn, J. R. & Shumway, N. P. J. exp. Med. 99, 275–282 (1954).
Yang, H. Y. T., Erdös, E. G. & Levin, Y. Biochim. biophys. Acta 214, 374–376 (1970).
Ondetti, M. A., Rubin, B. & Cushman, D. W. Science 196, 441–444 (1977).
Cushman, D. W., Cheung, H. S., Sabo, E. F. & Ondetti, M. A. Biochemistry 16, 5484–5491 (1977).
Levine, W. G. in The Pharmacological Basis of Therapeutics 5th edn (eds Goodman, L. S. & Gilman, A.) 919–920 (Macmillan, New York, 1975).
Cushman, D. W., Cheung, H. S., Sabo, E. F. & Ondetti, M. A. Fedn Proc. 33, 2778–2782 (1979).
Soffer, R. L. A. Rev. Biochem. 45, 73–94 (1976).
Cheung, H. S., Wang, F. L., Ondetti, M. A., Sabo, E. F. & Cushman, D. W. J. biol. Chem. 255, 401–407 (1980).
Beckner, C. F. & Caprioli, R. M. Biochem. biophys. Res. Commun. 93, 1290–1296 (1980).
Stability Constants of Metal Ion Complexes 520 (Spec. Publ. no. 17, The Chemical Society, London, 1964).
Wolfenden, R. A Rev. Biophys. Bioengng 5, 271–306 (1976).
Sweet, C. S. et al. J. Pharmac. exp. Ther. (submitted).
Gross, D. M. et al. J. Pharmac. exp. Ther. (submitted).
Biollaz, J. et al. Clin. Pharmac. Ther. (submitted).
Dorer, F. E., Kahn, J. R., Lentz, K. E., Levine, M. & Skeggs, L. T. Circulation Res. 34, 824–827 (1974).
Piquilloud, Y., Reinharz, A. & Roth, M. Biochim. biophys. Acta 206, 136–142 (1970).
Allinger, N. L. J. Am. chem. Soc. 81, 232 (1959).
Rubin, B. et al. J. Pharmac. exp. Ther. 204, 271 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Patchett, A., Harris, E., Tristram, E. et al. A new class of angiotensin-converting enzyme inhibitors. Nature 288, 280–283 (1980). https://doi.org/10.1038/288280a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/288280a0
- Springer Nature Limited
This article is cited by
-
A chemical technologist’s view on import substitution of medicines
Russian Chemical Bulletin (2023)
-
New Palladium-Containing Polymer Catalysts for the Synthesis of Enalapril
Pharmaceutical Chemistry Journal (2023)
-
Enzymatic Production of Two Tri-peptides on ACE-I Inhibition and Antioxidant Activities
International Journal of Peptide Research and Therapeutics (2020)
-
Hydrogenation N-alkylation of α-alanyl-α-proline with ethyl 2-oxo-4-phenylbutanoate on organometallic catalysts
Russian Chemical Bulletin (2020)
-
Development of membrane electrodes for selective determination of lisinopril in pharmaceuticals
Journal of Analytical Science and Technology (2019)