Abstract
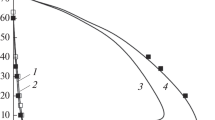
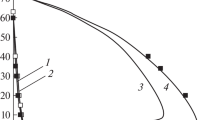
THE pollutant nitric oxide originates in combustion systems from nitrogen in the air or the fuel. Two processes have been identified whereby atmospheric N2 produces NO. The Zel'dovich1 mechanism has the rate-determining step O+N2→N+NO and is affected by oxygen atoms having concentrations2,3 above those for equilibrium. Fenimore's ‘prompt’ scheme4 is an attack on N2 by some hydrocarbon fragment with a lifetime of several µs in the flame's reaction zone5. Probable6 primary steps are  The oxidation of HCN and CN to NO has been discussed elsewhere6,7. We have burnt premixed H2–O2–N2 flames and measured the ‘prompt’ NO resulting from trace additions of hydrocarbons to the burner supplies. We found that ‘prompt’ NO yield is proportional to the number of carbon atoms in the hydrocarbon molecule. We consider that the hydrocarbons fragment into species with one carbon atom, in, or close to, the preheating region. Thus formation of ‘prompt’ NO, soot, ions in flames, and CH radiation from hydrocarbons all show related behaviour.
The oxidation of HCN and CN to NO has been discussed elsewhere6,7. We have burnt premixed H2–O2–N2 flames and measured the ‘prompt’ NO resulting from trace additions of hydrocarbons to the burner supplies. We found that ‘prompt’ NO yield is proportional to the number of carbon atoms in the hydrocarbon molecule. We consider that the hydrocarbons fragment into species with one carbon atom, in, or close to, the preheating region. Thus formation of ‘prompt’ NO, soot, ions in flames, and CH radiation from hydrocarbons all show related behaviour.
Similar content being viewed by others
References
Zel'dovich, Ya. B. Acta Phys. Chim. USSR 21, 577 (1946).
Bulewicz, E. M., James, C. G. & Sugden, T. M. Proc. R. Soc. A235, 89 (1956).
Bulewicz, E. M. & Sugden, T. M. Chem. Soc. Spec. Publ. No. 9, 81 (1958).
Fenimore, C. P. 13th Int. Symp. Combust. 373 (1971).
Gaydon, A. G. & Wolfhard, H. G. Flames, their Structure, Radiation and Temperature 3rd edn (Chapman and Hall, London, 1970).
Hayhurst, A. N. & McLean, H. A. G. Nature 251, 303 (1974).
Morley, C. Combust. Flame 27, 189 (1976).
Padley, P. J. & Sugden, T. M. Proc. R. Soc. A, 248, 248 (1958).
Padley, P. J. thesis, Univ. Cambridge (1959).
Jenkins, D. R. & Sugden, T. M. Flame Emission and Atomic Absorption Spectrometry 1 (eds Dean, J. A. & Rains, T. C.) 151 (Dekker, New York, 1959).
JANAF Thermochemical Tables 2nd edn (Nat. Bur. Stds, Washington, 1970).
Bradley, J. N. & West, K. O. J. chem. Soc. Faraday Trans. I, 72, 8 (1976); Bradley, J. N. Proc. R. Soc. A, 337, 199 (1974).
Braun, W., McNesby, J. R. & Bass, A. M. J. chem. Phys. 46, 2071 (1967).
Ferguson, R. E. Combust. Flame 1, 431 (1957).
Bulewicz, E. M. & Padley, P. J. 9th Int. Symp. Combust. 638 (1963).
Green, J. A. & Sugden, T. M. 9th Int. Symp. Combust. 607 (1963).
Blades, A. T. Can. J. Chem. 54, 2919 (1976) and references therein.
Peeters, J., Lambert, J. F., Hertoghe, P. & Van Tiggelen, A. 13th Int. Symp. Combust. 321 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HAYHURST, A., VINCE, I. Production of ‘prompt’ nitric oxide and decomposition of hydrocarbons in flames. Nature 266, 524–525 (1977). https://doi.org/10.1038/266524a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/266524a0
- Springer Nature Limited