Abstract
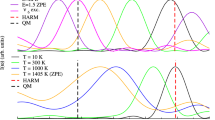
ULTRASONIC and spectroscopic techniques have shown that nitric oxide (X2Π) has an abnormally high probability of vibrational relaxation in self collisions at about 300° K. According to Nikitin, the process is electronically non-adiabatic1 because of a resonance between the 1Σ+g state of the vibrationless (NO)2 collision complex and the 3Σ−g state with one NO molecule vibrationally excited. With the point of resonance at a potential energy of about 2.8 kcalories/mole in the 3Σ−g complex, the theory predicts that the probability of relaxation should decrease sharply as the temperature is decreased below 300° K. To test this prediction, we have measured the rate of vibrational relaxation down to 100° K and we find, contrary to the prediction, that the temperature coefficient is negative.
Similar content being viewed by others
References
Nikitin, E. E., Optics Spectrosc., 9, 8 (1960).
Basco, N., Callear, A. B., and Norrish, R. G. W., Proc. Roy. Soc., A, 260, 459 (1961); Callear, A. B., Disc. Faraday Soc., 33, 28 (1962).
Callear, A. B., Pilling, M. J., and Smith, I. W. M., Trans. Faraday Soc., 64, 296 (1968).
Bauer, H. J., Kneser, H. O., and Sittig, E., J. Chem. Phys., 30, 1119 (1959).
Robben, F., J. Chem. Phys., 31, 420 (1959).
Wray, W. L., J. Chem. Phys., 36, 2597 (1962).
Lambert, J. D., and Salter, R., Proc. Roy. Soc. A, 243, 78 (1957); Corran, P. G., Lambert, S. D., Salter, R., and Warburton, B., Proc. Roy. Soc., A, 244, 212 (1958); Shields, F. D., J. Chem. Phys., 46, 1063 (1967).
Shin, H. K., J. Amer. Chem. Soc., 90, 3029 (1968).
Hirschfelder, J. O., Curtiss, C. F., and Bird, R. B., Molecular Theory of Gases and Liquids (Wiley, New York, 1954).
Jackson, J. M., and Mott, N. F., Proc. Roy. Soc., A, 137, 703 (1932).
Schwartz, R. N., Slawsky, Z. I., and Herzfeld, K. E., J. Chem. Phys., 20, 1591 (1952); Herzfeld, K. F., and Litovitz, T. A., Absorption and Dispersion of Ultrasonic Waves (Academic Press, New York, 1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BILLINGSLEY, J., CALLEAR, A. Negative Temperature Coefficient of the Vibrational Relaxation of Nitric Oxide: an Orientation Effect. Nature 221, 1136–1137 (1969). https://doi.org/10.1038/2211136a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/2211136a0
- Springer Nature Limited
This article is cited by
-
Vibrational Relaxation of Nitric Oxide at 500–1,900 K
Nature (1969)
-
Vibrational Relaxation of Nitric Oxide in Ternary Collisions
Nature (1969)