Abstract
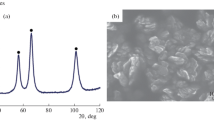
DURING recent studies of photo-adsorption on high area nickel oxide, decomposition of the oxide was observed at surprisingly low temperatures. Teichner1 first prepared this nickel oxide by decomposing Ni(OH)2 at 200° C for 2 h at a pressure of less than 10−5 mm mercury. It is yellow-green, with a surface area of approximately 100 m2g−1 and has a composition which is almost stoichiometric2. Both composition and area are little changed by further annealing under the same conditions, but on exposing the oxide to higher pressures of oxygen, it blackens instantly.
Similar content being viewed by others
References
Teichner, S. J., and Morrison, J. A., Trans. Farad. Soc., 51, 961 (1955).
Cotton, J. D., and Fensham, P. J., Trans. Farad. Soc., 59, 1444 (1963).
Imoto, T., Haramo, Y., and Nichi, Y., J. Chem. Soc. (Japan), 86, 694 (1965).
Fricke, R., Lehrmann, O., and Wolf, W., Z. Physik. Chemie (B), 37, 60 (1937).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LARKINS, F., FENSHAM, P. Decomposition of Nickel Oxide. Nature 215, 1268–1269 (1967). https://doi.org/10.1038/2151268a0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1038/2151268a0
- Springer Nature Limited
This article is cited by
-
Novel Ni cermets for anode-supported proton ceramic fuel cells
Journal of Solid State Electrochemistry (2019)