Abstract
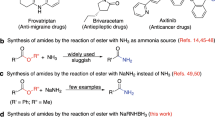
WE have recently introduced the trisdialkylaminoboranes as new reagents for the synthesis of enamines and tertiary amides1. Similarly, the trismonoalkylaminoboranes react with ketones to yield Schiff's bases, and with carboxylic acids to give secondary amides.
Similar content being viewed by others
References
Nelson, P., and Pelter, A., J. Chem. Soc., 5142 (1965).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LEVITT, T., PELTER, A. Trismonoalkylaminoboranes in Organic Synthesis: the Exploitation of a New Type of ‘Activated Ester’. Nature 211, 299–300 (1966). https://doi.org/10.1038/211299a0
Issue Date:
DOI: https://doi.org/10.1038/211299a0
- Springer Nature Limited
This article is cited by
-
Efficient synthesis of primary and secondary amides via reacting esters with alkali metal amidoboranes
Nature Communications (2021)
-
Organoboron compounds 330. Cyclic coordination compounds of boron from 2-aminopyridine and carbonyl compounds
Bulletin of the Academy of Sciences of the USSR Division of Chemical Science (1977)