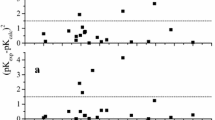
Abstract
MOST of the common acidic compounds can be classed as derivatives of the hydrides:  Within any of these classes, large differences in the free energies of ionization (ΔG0 = − RT logeK for the reaction BH → B− + H+ in dilute aqueous solution at 25° C.) can usually be attributed1,2 to the presence of charges in the near neighbourhood of the dissociating proton. Each charge on the atom next to the one bearing the dissociating proton will decrease the value of ΔG0 by about 14 kgm.cal. if positive and will increase ΔG0 by about 14 kgm.cal. if negative. The effect of a charge upon ΔG0 is reduced when it is situated farther from the dissociating proton and ± 14(Q/2n kgm.cal. has to be added to ΔG0 to account for the effect of a charge, where n is the number of atoms between the atom bearing the charge and the dissociating proton2,3.
Within any of these classes, large differences in the free energies of ionization (ΔG0 = − RT logeK for the reaction BH → B− + H+ in dilute aqueous solution at 25° C.) can usually be attributed1,2 to the presence of charges in the near neighbourhood of the dissociating proton. Each charge on the atom next to the one bearing the dissociating proton will decrease the value of ΔG0 by about 14 kgm.cal. if positive and will increase ΔG0 by about 14 kgm.cal. if negative. The effect of a charge upon ΔG0 is reduced when it is situated farther from the dissociating proton and ± 14(Q/2n kgm.cal. has to be added to ΔG0 to account for the effect of a charge, where n is the number of atoms between the atom bearing the charge and the dissociating proton2,3.
Similar content being viewed by others
References
Brönsted, J. N., J. Phys. Chem., 30, 777 (1926).
McGowan, J. C., Nature, 159, 644 (1947).
McGowan, J. C., Chem. and Indust., 632 (1948).
Brönsted, J. N., and Volquartz, K., Z. phys. Chem., 134, 97 (1928).
Sidgwick, N. V., “The Chemical Elements and Their Compounds”, 1, 1009 (Oxford, 1950).
Arden, T. V., J. Chem. Soc., 350 (1951).
Lamb, A. B., and Jacques, A. G., J. Amer. Chem. Soc., 60, 1215 (1938).
Bray, W. C., and Hershey, A. V., J. Amer. Chem. Soc., 56, 1889 (1934).
Davies, C. W., and Hoyle, B. E., J. Chem. Soc., 233 (1951).
Bell, R. P., and Prue, J. E., J. Chem. Soc., 362 (1949).
Davies, C. W., J. Chem. Soc., 349 (1939).
Stock, D. I., and Davies, C. W., Trans. Farad. Soc., 44, 856 (1948).
Pauling, L., “The Nature of the Chemical Bond”, 64 (2nd edit., New York and London, 1948).
Emeléus, H. J., and Anderson, J. S., “Modern Aspects of Inorganic Chemistry”, 142, 143 and 155 (London, 1943).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McGOWAN, J. Estimation of the Approximate Ionization Constants of Inorganic Bases. Nature 168, 601–602 (1951). https://doi.org/10.1038/168601a0
Issue Date:
DOI: https://doi.org/10.1038/168601a0
- Springer Nature Limited