Abstract
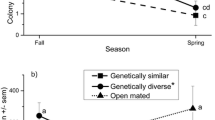
In many species of animals, females typically mate with more than one male (polyandry). Some social insects carry this behaviour to extremes1. For example, honeybee queens mate with ten to twenty (or even more) males on their nuptial flights2. The reasons for this behaviour remain unknown, given the obvious costs of time, energy and exposure to predation. Several potential benefits of polyandry have been proposed1,3,3,4, but none are well supported yet. Here we test the hypothesis that genetic diversity among a female's offspring may offer some protection from parasitism5,6,7. We artificially inseminated queens of a bumble-bee (Bombus terrestris L.) with sperm of either low or high genetic diversity. The resulting colonies were exposed to parasitism under field conditions. High-diversity colonies had fewer parasites and showed greater reproductive success, on average, than did low-diversity colonies. We suggest that female mating frequency may be influenced in part by parasites.



Similar content being viewed by others
References
Boomsma, J. J. & Ratnieks, F. L. W. Paternity in eusocial hymenoptera. Phil. Trans. R. Soc. Lond. B 351, 947–975 (1996).
Moritz, F. R. A. et al. High degree of polyandry in Apis dorsata queens detected by DNA microsatellite variability. Behav. Ecol. Sociobiol. 37, 357–363 (1995).
Boomsma, J. J. & Grafen, A. Colony-level sex ratio selection in the eusocial Hymenoptera. J. Evol. Biol. 3, 383–407 (1991).
Keller, L. & Reeve, H. K. Partitioning of reproduction in animal societies. TREE 9, 98–102 (1994).
Tooby, J. Pathogens, polymorphism, and the evolution of sex. J. Theor. Biol. 97, 557–576 (1982).
Hamilton, W. D. in Animal Societies: Theory and Facts (eds Itô, Y., Brown, J. L. & Kikkawa, J.) 81–102 (Japan. Sci. Soc. Press, Tokyo, (1987)).
Sherman, P. W., Seeley, T. D. & Reeve, H. K. Parasites, pathogens, and polyandry in social Hymenoptera. Am. Nat. 131, 602–610 (1988).
Kulincevic, J. M. in Bee Genetics and Breeding (ed. Rinderer, T. E.) 391–414 (Academic, Orlando, (1986)).
Shykoff, J. A. & Schmid-Hempel, P. Parasites and the advantage of genetic variability within social insect colonies. Proc. R. Soc. Lond. B 243, 55–58 (1991).
Schmid-Hempel, P. & Loosli, R. Acontribution to the knowledge of Nosema infections in bumble bees, Bombus spp. Apidologie (in the press).
Schmid-Hempel, P. Infection and colony variability in social insects. Phil. Trans. R. Soc. Lond. B 346, 313–321 (1994).
Crozier, R. H. & Pamilo, P. Evolution of Social Insect Colonies 1–306 (Oxford Univ. Press, (1996)).
Durrer, S. & Schmid-Hempel, P. Shared use of flowers leads to horizontal pathogen transmission. Proc. R. Soc. Lond. B 258, 299–302 (1994).
Estoup, A., Scholl, A., Pouvreau, A. & Solignac, M. Monandry and polyandry in bumble bees (Hymenoptera, Bombinae) as evidenced by highly variable microsatellites. Mol. Ecol. 4, 89–93 (1995).
Röseler, P. -F. Die Anzahl Spermien im Receptaculum seminis von Hummelköniginnen (Hymenoptera, Apidae, Bombinae). Apidologie 4, 267–274 (1973).
Thorén, P., Estoup, A., Paxton, R. J. & Tengö, J. in 6th Congr. Eur. Soc. Evol. Biol. Arnhem S3 (Eur. Soc. Evol. Biol., Arnhem, (1997)).
MacFarlane, R. P., Lipa, J. J. & Liu, H. J. Bumble bee pathogens and internal enemies. Bee World 76, 130–148 (1995).
Schmid-Hempel, P. Parasites in Social Insects (Princeton Univ. Press, Princeton, (1998)).
Shykoff, J. A. & Schmid-Hempel, P. Parasites delay worker reproduction in bumblebees: consequences for eusociality. Behav. Ecol. 2, 242–248 (1991).
Müller, C. B. & Schmid-Hempel, P. Variation in worker mortality and reproductive performance in the bumble bee, B. lucorum. Funct. Ecol. 6, 48–56 (1992).
Müller, C. B. & Schmid-Hempel, P. Correlates of reproductive success among field colonies of Bombus lucorum L.: the importance of growth and parasites. Ecol. Entomol. 17, 343–353 (1993).
Liersch, S. & Schmid-Hempel, P. Genetic variability within social insect colonies reduces parasite load. Proc. R. Soc. Lond. B 265, 221–225 (1998).
Walker, W. F. Sperm utilization strategies in non-social insects. Am. Nat. 115, 780–799 (1980).
Madsen, T., Shine, R., Loman, J. & Hakensson, T. Why do female adders copulate so frequently? Nature 355, 440–441 (1992).
Acknowledgements
We thank K. Boomsma, M. Brown, F. Fischer, B. Imhoof, S. Koulianos, N. Krüger, E.Magro, E. Meier, R. E. Page Jr, F. Ratnieks, R. Schmid-Hempel and H. Schwarz for help and comments, and the local communities and forestry commissions for use of their land. This work was supported bygrants to P.S.-H. from the Swiss National Science Foundation and the Swiss Office of Science and Technology (within a European TMR network).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baer, B., Schmid-Hempel, P. Experimental variation in polyandry affects parasite loads and fitness in a bumble-bee. Nature 397, 151–154 (1999). https://doi.org/10.1038/16451
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/16451
- Springer Nature Limited
This article is cited by
-
Genetic diversity, paternal origin and pathogen resistance in Cataglyphis desert ants
Behavioral Ecology and Sociobiology (2023)
-
Decoupled evolution of mating biology and social structure in Acromyrmex leaf-cutting ants
Behavioral Ecology and Sociobiology (2022)
-
Direct evidence for increased disease resistance in polyandrous broods exists only in eusocial Hymenoptera
BMC Ecology and Evolution (2021)
-
Experimental increase of worker diversity benefits brood production in ants
BMC Ecology and Evolution (2021)
-
Gram-negative bacteria associated with a dominant arboreal ant species outcompete phyllosphere-associated bacteria species in a tropical canopy
Oecologia (2021)