Abstract
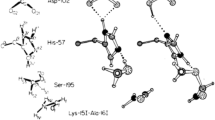
THE trigonal planar symmetry of the three bonds formed by the nitrogen atom attached to the sulphur atom in the structure described in the preceding communication suggests that the orbitals of this nitrogen atom correspond in bond formation to a hybridization of the type (1s)2(2p)2(2s2p2)3 rather than to (1s)2(2s)22px2py2pz. In the former electronic configuration, the three sp2 orbitals will possess trigonal symmetry, and there will be one doubly occupied π-orbital orthogonal to the plane of the sp2-orbitals. This type of hybridization has been postulated by us1 in a discussion of the properties of the >N—H O=C< structure in proteins.
Similar content being viewed by others
References
Evans, M. G., and Gergely, J. (in the press).
Mulliken, R., J. Chem. Phys., 2, 782 (1934).
Gordy, W., J. Chem. Phys., 15, 81 (1947).
Costain, W., and Cox, E. G., Nature, 160, 826 (1947).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
EVANS, M., GERGELY, J. [Letters to Editor]. Nature 162, 770–771 (1948). https://doi.org/10.1038/162770b0
Issue Date:
DOI: https://doi.org/10.1038/162770b0
- Springer Nature Limited