Abstract
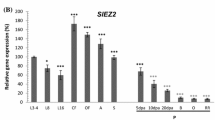
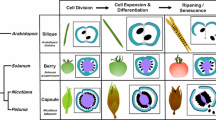
The SELF PRUNING (SP) gene controls the regularity of the vegetative-reproductive switch along the compound shoot of tomato and thus conditions the `determinate' (sp/sp) and `indeterminate' (SP −) growth habits of the plant. SP is a developmental regulator which is homologous to CENTRORADIALIS (CEN) from Antirrhinum and TERMINAL FLOWER 1 (TFL1) and FLOWERING LOCUS T (FT) from Arabidopsis. Here we report that SP is a member of a gene family in tomato composed of at least six genes, none of which is represented in the tomato EST collection. Sequence analysis of the SP gene family revealed that its members share homology along their entire coding regions both among themselves and with the six members of the Arabidopsis family. Furthermore, members of the gene family in the two species display a common genomic organization (intron-exon pattern). In tomato, phylogenetically close homologues diverged considerably with respect to their organ expression patterns while SP2I and its closest homologue from Arabidopsis (MFT) exhibited constitutive expression. This reserarch focusing on a plant of sympodial growth habit sets the stage for a functional analysis of this weakly expressed gene family which plays a key role in determining plant architecture.
Similar content being viewed by others
References
Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W. and Lipman, D.J. 1997. Gapped BLAST and PSIBLAST: a new generation of protein database search programs. Nucl. Acids Res. 25: 3389–3402.
Alvarez, J., Guli, C.L. and Yx, D.R.S. 1992. Terminal flower: a gene affecting inflorescence development in Arabidopsis thaliana. Plant J. 2: 103–116.
Amaya, I., Ratcliffe, O.J. and and Bradley, D.J. 1999. Expression of CENTRORADIALIS (CEN) and CEN-like genes in tobacco reveals a conserved mechanism controlling phase change in diverse species. Plant Cell 11: 1405–1417.
Atherton, J.G. and Harris, G.P. 1986. Flowering. In: J.G. Atherton, and J. Rudich (Eds.) The Tomato Crop: A Scientific Basis for Improvement, Chapman and Hall, London, pp. 171–173.
Banfield, M.J. and Brady, R.L. 2000. The structure of Antirrhinum centroradialis protein (CEN) suggests a role as a kinase regulator. J. Mol. Biol. 297: 1159–1170.
Banfield, M.J., Barker, J.J., Perry, A.C. and Brady, R.L. 1998. Function from structure? The crystal structure of human phosphatidylethanolamine-binding protein suggests a role in membrane signal transduction. Structure 6: 1245–1254.
Bell, P.R. 1992. Green Plants: Their Origin and Diversity. Cambridge University Press, Cambridge, UK.
Bradley, D., Carpenter, R., Copsey, L., Vincent, C., Rothstein, S. and Coen, E. 1996. Control of inflorescence architecture in Antirrhinum. Nature 379: 791–797.
Bradley, D., Ratcliffe, O., Vincent, C., Carpenter, R. and Coen, E. 1997. Inflorescence commitment and architecture in Arabidopsis. Science 275: 80–83.
Cronquist, A. 1988. The Evolution and Classification of Flowering Plants. Allen Press, Lawrence, KS.
Fridman, E., Liu, Y.S., Carmel-Goren, L., Gur, A., Shoresh, M., Pleban, T., Eshed, Y. and Zamir, D. 2002. Two tightly linked QTLs modify tomato sugar content via different physiological pathways. Mol. Genet. Genomics 266: 821–826.
Grandy, D.K., Hanneman, E., Bunzow, J., Shih, M., Machida, C.A., Bidlack, J.M. and Civelli, O. 1990. Purification, cloning and tissue distribution of a 23-kDa rat protein isolated by morphine affinity chromatography. Mol. Endocrinol. 4: 1370–1376.
Hareven, D., Gutfinger, T., Parnis, A., Eshed, Y. and Lifschitz, E. 1996. The making of a compound leaf: genetic manipulation of leaf architecture in tomato. Cell 84: 735–744.
Kardailsky, I., Shukla, V.K., Ahn, J.H., Dagenais, N., Christensen, S.K., Nguyen, J.T., Chory, J., Harrison, M.J. and Weigel, D. 1999. Activation tagging of the floral inducer FT. Science 286: 1962–1965.
Kobayashi, Y., Kaya, H., Goto, K., Iwabuchi, M. and Araki, T. 1999. A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962.
Ku, H.M., Vision, T., Liu, J. and Tanksley, S.D. 2000. Comparing sequenced segments of the tomato and Arabidopsis genomes: large-scale duplication followed by selective gene loss creates a network of synteny. Proc. Natl. Acad. Sci. USA 97: 9121–9126.
Mimida, N., Goto, K., Kobayashi, Y., Araki, T., Ahn, J.H., Weigel, D., Murata, M., Motoyoshi, F. and Sakamoto, W. 2001. Functional divergence of the TFL1-like gene family in Arabidopsis revealed by characterization of a novel homologue. Genes Cells 6: 327–336.
Ohshima, S., Murata, M., Sakamoto, W., Ogura, Y. and Motoyoshi, F. 1997. Cloning and molecular analysis of the Arabidopsis gene Terminal Flower 1. Mol. Gen. Genet. 254: 186–194.
Page, R.D.M. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Computer applications. Biosciences 12: 357–358.
Pan, Q., Liu, Y. S., Budai-Hadrian, O., Sela, M., Carmel-Goren, L., Zamir, D. and Fluhr, R. 2000. Comparative genetics of nucleotide binding site: leucine rich repeat resistance gene homologues in the genomes of two dicotyledons: tomato and arabidopsis. Genetics 155: 309–322.
Parnis, A., Cohen, O., Gutfinger, T., Hareven, D., Zamir, D. and Lifschitz, E. 1997. Two different developmental mutants of tomato, Mouse ear and Curl, are associated with two distinct modes of abnormal transcriptional regulation of a KNOTTED gene. Plant Cell 9: 2143–2158.
Pnueli, L., Carmel-Goren, L., Hareven, D., Gutfinger, T., Alvarez, J., Ganal, M., Zamir, D. and Lifschitz, E. 1998. The SELFPRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development 125: 1979–1989.
Pnueli, L., Gutfinger, T., Hareven, D., Ben-Naim, O., Ron, N. and Adir, N. 2001. Tomato SP-interacting proteins define a conserved signaling system that regulates shoot architecture and flowering. Plant Cell 13: 2687–2702.
Saitou, N. and Nei, M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425.
Schoentgen, F. and Jolles, P. 1995. From structure to function: possible biological roles of a new widespread protein family binding hydrophobic ligands and displaying a nucleotide binding site. FEBS Lett. 369: 22–26.
Serre, L., Vallee, B., Bureaund, N., Schoentgen, F. and Zelwer, C. 1998. Crystal structure of the phosphatidylethanolaminebinding protein from bovine brain: a novel structural class of phospholipid-binding proteins. Structure 6: 1255–1265.
Serre, L., Pereira de Jesus, K., Zelwer, C., Bureaud, N., Schoentgen, F. and Benedetti, H. 2001. Crystal structures of YBHB and YBCL from Escherichia coli, two bacterial homologues to a Raf kinase inhibitor protein. J. Mol. Biol. 310: 617–634.
Shannon, S. and Meeks-Wagner, D. 1991. A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell 3: 877–892.
Simister, P.C., Banfield, M.J., and Brady R.L. 2002. The crystal structure of PEBP-2, a homologue of the PEBP/RKIP family. Acta Crystallogr. D Biol. Crystallogr. 58: 1077–1080.
Thompson, J.D., Higgins, D.G. and Gibson, T.J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl. Acids Res. 22: 4673–4680.
van der Hoeven, R., Ronning, C., Giovannoni, J., Martin, G. and Tanksley, S. 2002. Deductions about the number, organization, and evolution of genes in the tomato genome based on analysis of a large expressed sequence tag collection and selective genomic sequencing. Plant Cell 14: 1441–1456.
Yeager, A.F. 1927. Determinate growth in tomato. J. Hered. 18: 263–265.
Zamir, D. and Tanksley S.D. 1988. Tomato genome is comprised largely of fast evolving, low copy number sequences. Mol. Gen. Genet. 213: 254–261.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carmel-Goren, L., Liu, Y.S., Lifschitz, E. et al. The SELF-PRUNING gene family in tomato. Plant Mol Biol 52, 1215–1222 (2003). https://doi.org/10.1023/B:PLAN.0000004333.96451.11
Issue Date:
DOI: https://doi.org/10.1023/B:PLAN.0000004333.96451.11